Goldsmith Research Group
Auburn University, Department of Chemistry and Biochemistry
Research
Overview:
Research in the Goldsmith Lab focuses on developing redox-active coordination complexes and using these compounds to address health- and energy-related issues. One overarching principle is the use of ligand design to enhance or even fully introduce novel functionality to an inorganic compound. We currently have three active projects: 1) magnetic resonance imaging (MRI) contrast agent sensors for reactive oxygen species, 2) biomimetic catalysts for the degradation of superoxide and hydrogen peroxide, and 3) electrocatalysts for the reduction of dioxygen to water. Previously, we have also investigated non-heme iron compounds as catalysts for the oxidation of C-H bonds by H2O2 and O2 and gallium compounds as catalysts for olefin epoxidation. We collaborate extensively with researchers within and outside of Auburn University.
MRI Contrast Agent Sensors for Reactive Oxygen Species:
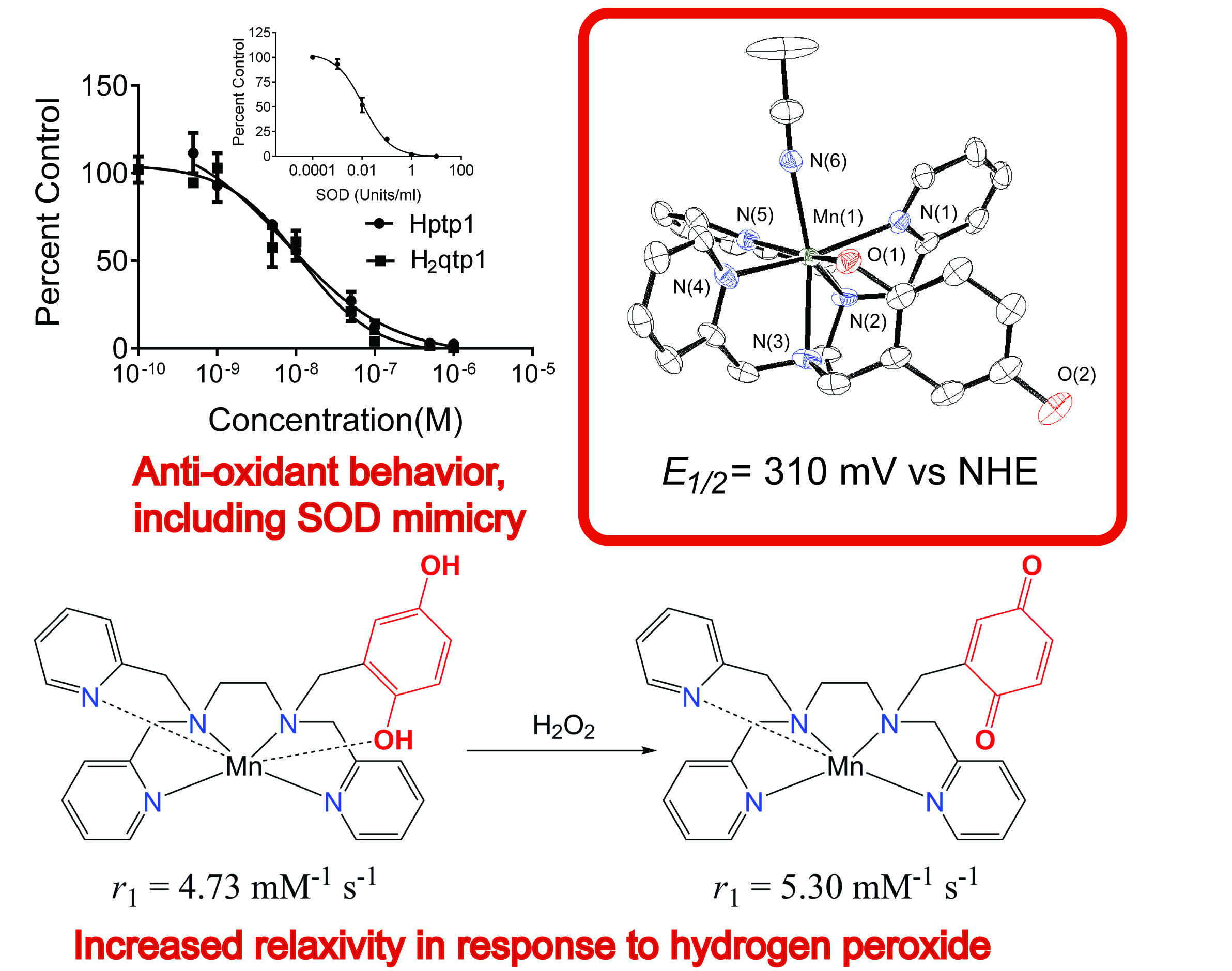
Reactive oxygen species, such as O2- anions, hydrogen peroxide molecules, and hydroxyl radicals, are intricately involved in many biological processes. The body needs to carefully manage their concentrations to maintain health. Reactive oxygen species can oxidize and degrade biomolecules, and excess amounts of these oxidants have been correlated to a wide array of inflammatory, cardiovascular, and neurological disorders (e.g. Parkinson's, Alzheimer's Diseases). Current methodology for monitoring and assessing oxidative stress relies on either indirect methods, such as analyzing protein and lipid oxidation, and/or invasive tissue sampling. The development of sensors that can directly assess oxidative activity within whole-body subjects could allow us to better understand the connections between reactive oxygen species and disease and potentially allow the earlier diagnosis and treatment of several lethal and debilitating disorders. Commonly available MRI instrumentation can detect such changes within thicker biological samples without the necessity of invasive measures, making it a highly attractive diagnostic technique. The primary goal of the project is to develop MRI contrast agents that exhibit changes in their relaxivity upon reaction with a reactive oxygen species. Recent publications from the Goldsmith Group have reported manganese(II) and iron(II) complexes with redox-active ligands that exhibit changes in their T1-derived relaxivity upon reaction with H2O2. The changes in the relaxivity allows the coordination complexes to act as sensors for H2O2. Notably, the responses do not require a co-analyte and are not triggered by O2. This work is being done in collaboration with the Auburn University MRI Research Center and the Auburn University College of Veterinary Medicine, was previously supported by a grant from AURIC, and is currently supported by the NSF. The below figure appears in a manuscript published in the Journal of the American Chemical Society, with two subsequent reports appearing in Inorganic Chemistry, including one that was a featured article, and a more recent article in Chemistry - A European Journal. The project has been highlighted by Auburn's College of Science and Mathematics.
Catalysts for Superoxide and Hydrogen Peroxide Degradation:
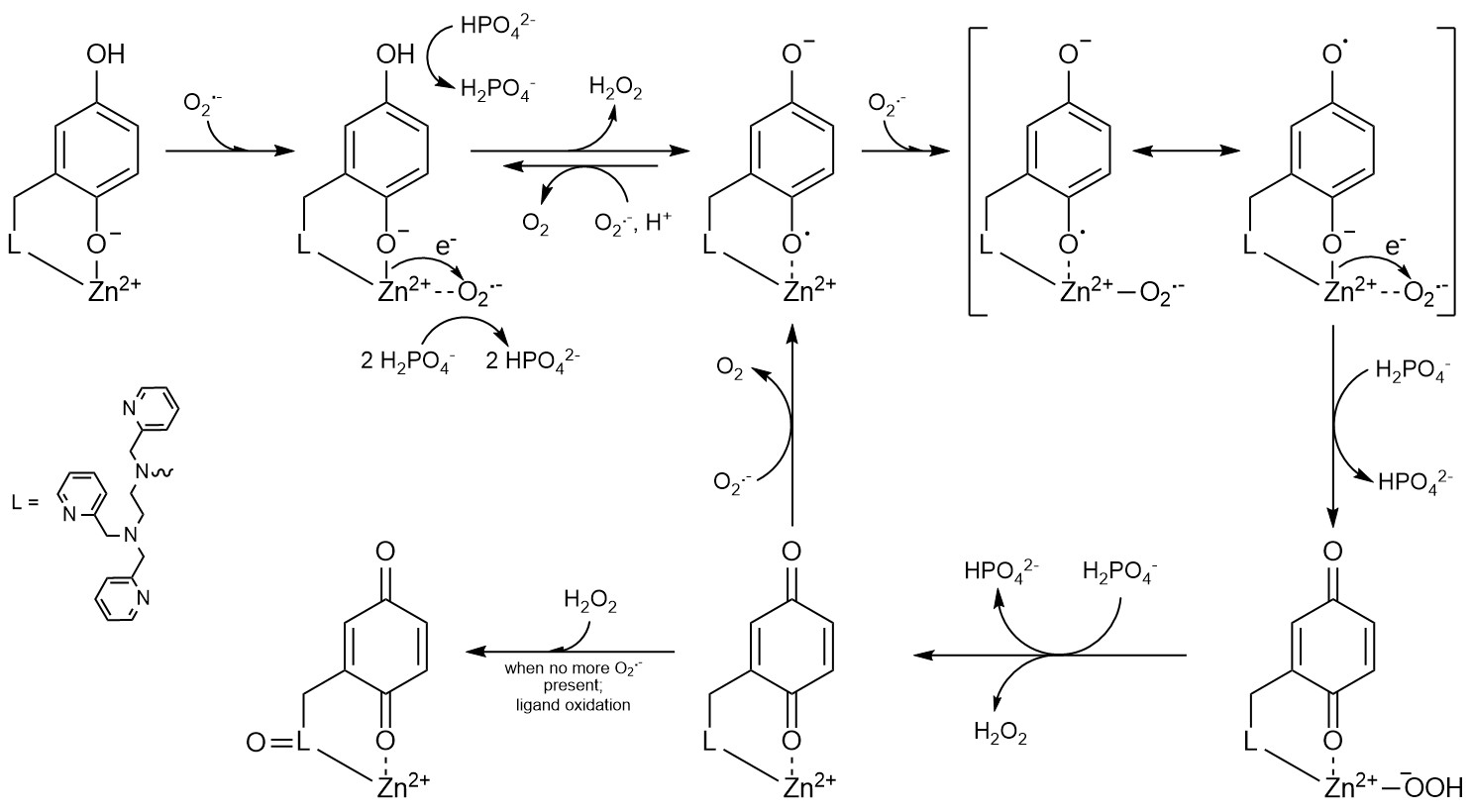
The likely involvement of reactive oxygen species in pathology has motivated considerable interest in developing antioxidants to control their concentrations. We found that the previously described MRI sensors display strong antioxidant activity, and these or related molecules may be useful for simultaneously imaging and treat oxidative stress in vivo. Studies on the antioxidant properties of our sensors has appeared in the Journal of the American Chemical Society and Chemical Science; this work was done in collaboration with Prof. Ivana Ivanović-Burmazović from the Ludwig Maximilian University of Munich. More recently, we found that the redox-active ligands used in our more recent sensors could serve as redox partners for O2-. Although the metal-free organic molecules themselves cannot catalyze O2- degradation, zinc(II) complexes with the ligands can. This work, which appeared in Nature Chemistry, Inorganic Chemistry, and the Journal of Physical Chemistry A, demonstrated that coordination complexes between redox-inactive metal ions and redox-active ligands can mimic the activity of superoxide dismutase. Recent work resulted from a collaboration between ourselves and Prof. Evangelos Miliordos of Auburn University. We are currently investigating how the choice of metal ion influences the antioxidant activity of coordination complexes with polydentate quinol-containing ligands. We find that in certain cases, the compounds catalyze the decomposition of H2O2 rather than O2-, thereby mimicking catalase instead of superoxide dismutase. Our inital study of these compounds' catalase activity was published in Chemical Science. Much like the superoxide dismutase mimics, the redox activity of the organic ligand allows redox-inactive metal ions to promote catalytic activity that was previously thought to be limited to redox-active metals.
Electrocatalysts for the Oxygen Reduction Reaction:
Oxygen reduction is a key reaction not only for biology but also for energy storage technology (fuel cells, metal-air batteries). In order to work most efficiently, the electrocatalysts for the oxygen reduction reaction (ORR) need to rapidly reduce O2 to water at a low overpotential without generating H2O2, which can damage the components of the battery or fuel cell. Recently, we have found that the redox-active quinols used in our MRI contrast agent sensors and antioxidants can improve the selectivity of ORR for water and allow previously inactive metal-ligand frameworks to act as electrocatalysts for this reaction. Our first article in this field appears in the Journal of the American Chemical Society. This work resulted from a successful collaboration with Prof. Byron Farnum and his research group.
