Department of Chemistry and Biochemistry
James E. Land Associate Professor
Research Areas: Physical
Office: 114 Extension Hall
Address:
179 Chemistry Building
Auburn, AL 36849
Phone: (334) 844-6957
Email: emiliord@auburn.edu
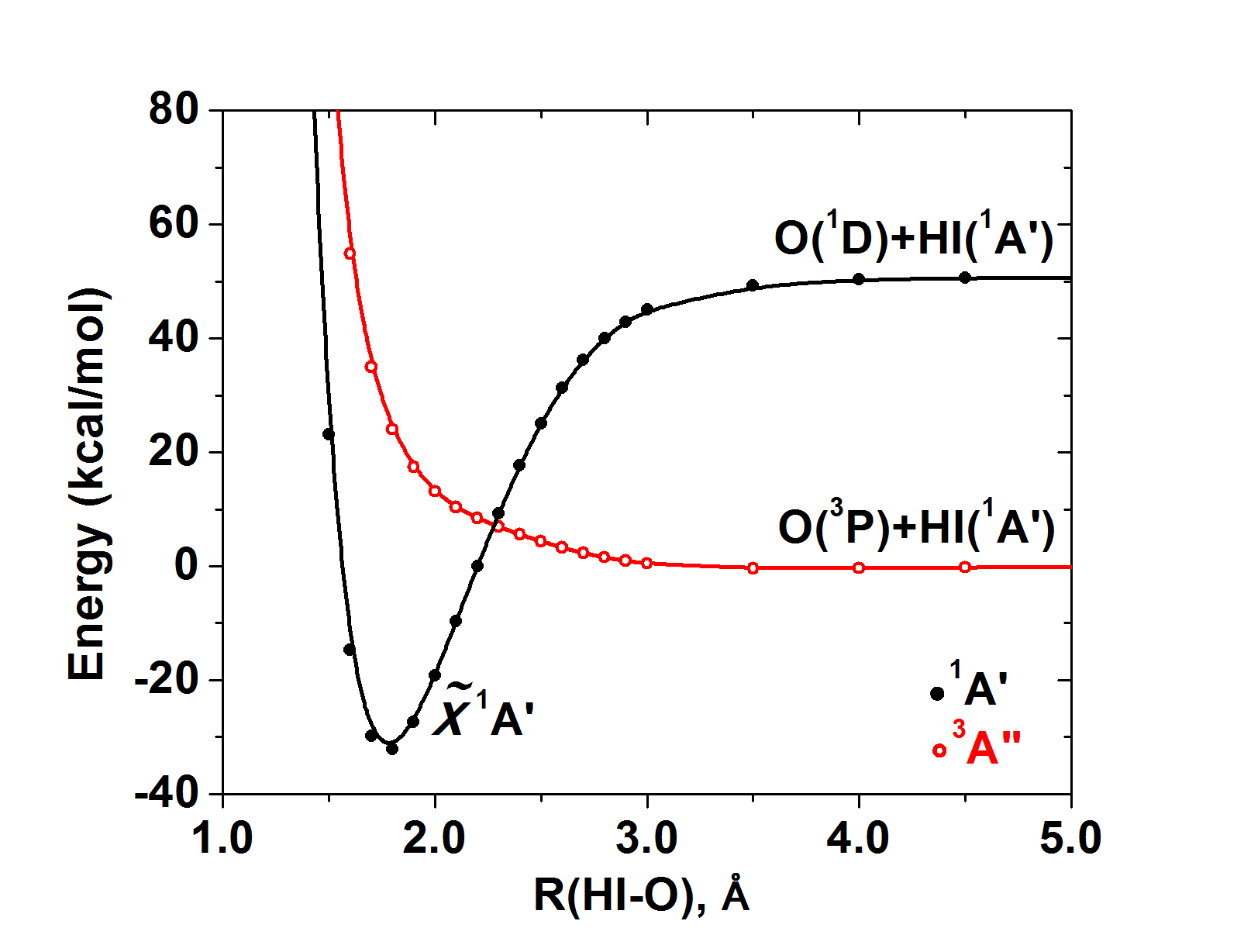
The construction of potential energy profiles along a reaction coordinate for the ground and excited electronic states provides the “blueprint” of the reactants chemical activity. Quite often the lowest energy molecular structure originates from the excited electronic states of the atoms and/or fragments composing it. For example, atomic oxygen participates in molecules with both its ground state 3P with two unpaired electrons (e.g. 1pz2 1px1 1py1) and its first excited state 1D which has components with no unpaired electrons (e.g. 1px2 1py2). An example of the first case is H2O, while examples of the second case are many good oxidants (such as N2O, PhIO) and several "hypervalent" systems (such as H2SO4). We recently studied the oxidation mechanism of alkenes by the model oxidant HIO, which is representative of PhIO, [Phys. Chem. Chem. Phys. 19, 18152 (2017)] and we proved that the good oxidation properties of such oxidants are mainly because of the fact that they involve an in situ excited oxygen atom, which is known to be very reactive. The following potential energy curves provide a clean picture that HIO is formed by the O(1D) state:
 Therefore the electronic structure transformations occuring during the oxidation reaction of alkenes are illustrated succinctly by the figure below:
Therefore the electronic structure transformations occuring during the oxidation reaction of alkenes are illustrated succinctly by the figure below:
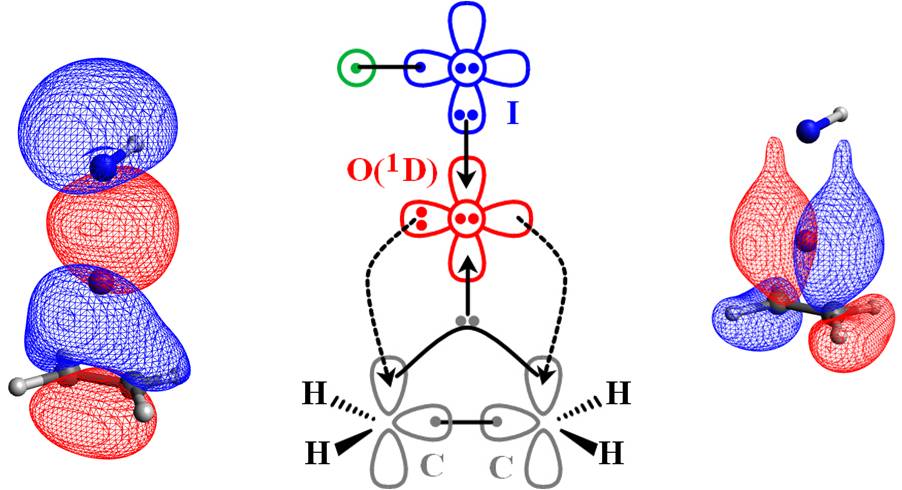
It is oxygen's excited state which facilitates this reaction. The involvement of excited states is very common in chemical reactions. In our group we make an effort to explain chemical processes at a very fundamental level which will enable us to better comprehend chemistry, predict molecular entities not previously reported in the literature, and propose more efficient catalytic reaction pathways. To cope with the complex multi-radical nature of excited states, we apply advanced computational methodologies such as multi-reference configuration interaction combined with large basis sets. Other computational approaches used in the lab are (but not limited to) coupled clusters, perturbation theory, density functional theory (DFT), periodic DFT calculations.
Predicting molecular species
We recently showed that the beryllium tetra-ammonia complexes Be(NH3)40,± can be described as a Be(NH3)42+ core with one, two, or three electrons orbiting around it residing at hydrogenic-type (s, p, d, f, ...) orbitals [J. Phys. Chem. Lett. 9, 84 (2018)]:
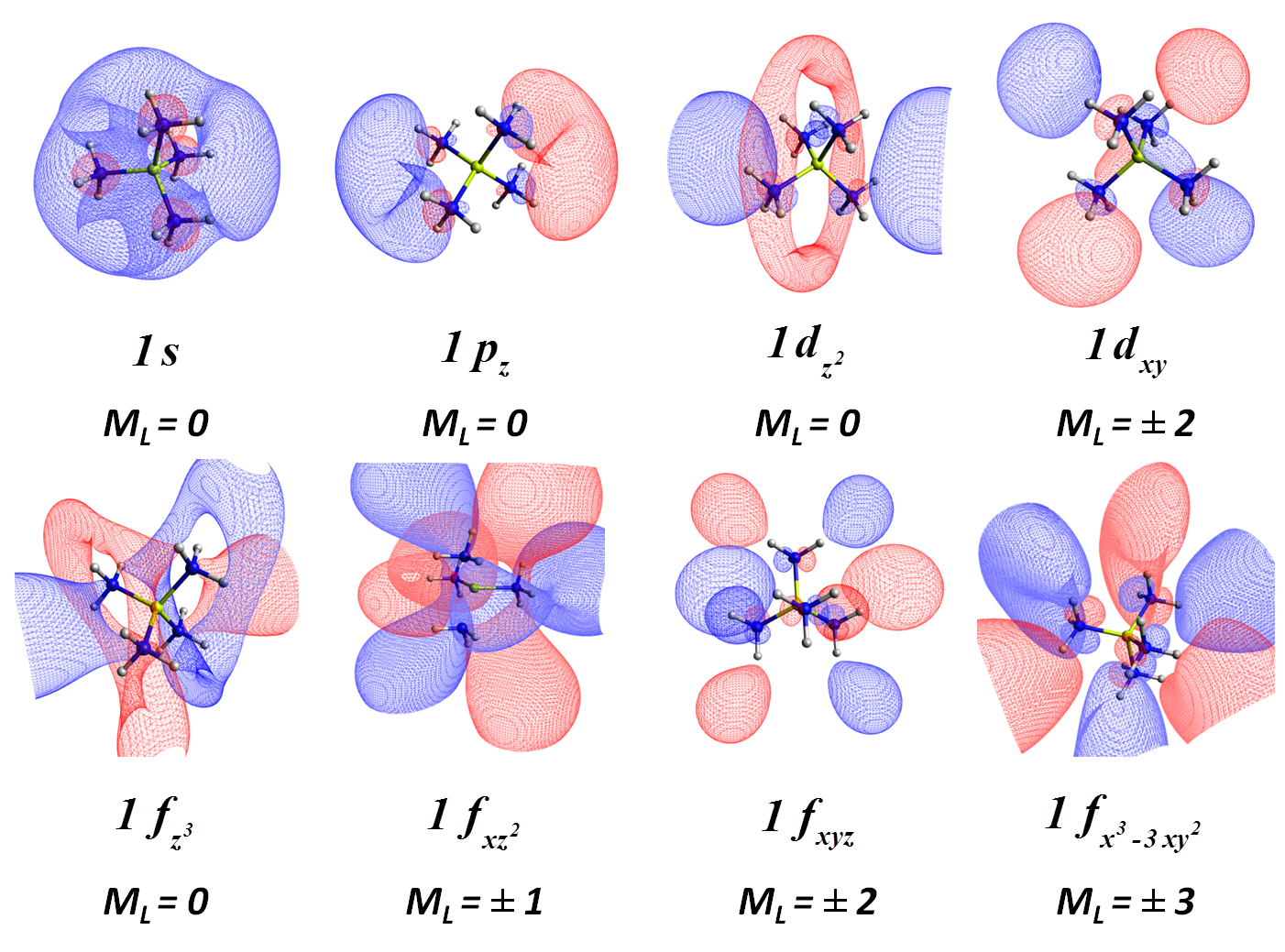 The majority of metals make similar complexes which we call solvated electron precursors (SEP). The Aufbau rules for SEPs differ from hydrogen-like atoms. Instead of the traditional 1s, 2s, 2p, 3s, 3p, 3d, ... orbitals series, the orbital model resembles more the nuclear shell or jellium models: 1s, 1p, 1d, 2s, 1f, 2p, ... SEPs can bind together forming aggregates exactly like atoms form molecules.Our theoretical calculations are able to predict the existence of novel materials (SEPs polymers or crystals). Transition-metal-based SEPs are expected to provide materials for magnetic applications.
The majority of metals make similar complexes which we call solvated electron precursors (SEP). The Aufbau rules for SEPs differ from hydrogen-like atoms. Instead of the traditional 1s, 2s, 2p, 3s, 3p, 3d, ... orbitals series, the orbital model resembles more the nuclear shell or jellium models: 1s, 1p, 1d, 2s, 1f, 2p, ... SEPs can bind together forming aggregates exactly like atoms form molecules.Our theoretical calculations are able to predict the existence of novel materials (SEPs polymers or crystals). Transition-metal-based SEPs are expected to provide materials for magnetic applications.
Proposing more efficient catalytic reaction pathways
The targeted reactions in our group pertain to the modern worldwide energy needs. We are highly interested in the reaction of small transition metal compounds with water and methane (homogeneous catalysis). Our ultimate goal is the understanding of the role of both the transition metals and ligands nature on the catalytic water splitting and C-H activation reactions.
The former reaction produces H2 which is considered a viable replacement of the conventional fossil fuels. The latter can aid at the chemical transformation of the inert hydrocarbons, which are abundant in natural gas and other fossil fuels. The light absorption often triggers the above catalytic cycles which dictates the investigation of reaction pathways for electronically excited states.
Comprehending chemistry better
We perform high-level theoretical chemistry calculations to provide interpretation of experimental observations based on the necessary fundamental physical principles.
Recently Dr. Anne Gorden's group (Auburn University - Department of Chemistry and Biochemistry) observed that thorium-napthylsalophen sandwich complexes exhibit unusual fluorescence, while the same complex of the isovalent cerium does not. We were able to find theoretically that thorium is lacking these electronic states which are responsible for quenching fluorescence [Chem. Comm. 53, 11984 (2017)].

We also collaborate with experimental groups within the department. For example, we have an ongoing collaboration with Dr. Ming Chen in the area of organic synthesis of assymetric compound, Dr. Christian Goldsmith on fascinating transition metal chemistry, and Dr. Anne Gorden on lanthanides and actinides chemistry.
Publications as PI at Auburn University
(For an updated list please direct your browser here)
2017
- S. N. Khan and E. Miliordos, “The role of O(1D) in the oxidation mechanism of ethylene by iodosobenzene and other hypervalent molecules”, Physical Chemistry Chemical Physics 19, 18152 (2017), DOI: 10.1039/c7cp04000h.
- I. R. Ariyarathna and E. Miliordos, “The versatile personality of beryllium: Be(O2)1-2 vs. Be(CO)1-2”, Journal of Physical Chemistry A 121, 7051 (2017), DOI: 10.1021/acs.jpca.7b06519.
- E. E. Hardy, K. M. Wyss, J. D. Gorden, I. R. Ariyarathna, E. Miliordos, and A. E. V. Gorden, “Th(IV) and Ce(IV) napthylsalophen sandwich complexes: Characterization of unusual thorium fluorescence in solution and solid phase”, Chemical Communications 53, 11984 (2017), DOI: 10.1039/C7CC06868A. Journal cover.
2018
- I. R. Ariyarathna, S. N. Khan, F. Pawłowski, J. V. Ortiz, and E. Miliordos, “Aufbau rules for solvated electron precursors: Be(NH3)40,± complexes and beyond”, Journal of Physical Chemistry Letters 9, 84 (2018), DOI: 10.1021/acs.jpclett.7b03000.
- I. R. Ariyarathna and E. Miliordos, “Ab initio investigation of the ground and excited states of MoO+,2+,- and their catalytic strength on water activation”, Physical Chemistry Chemical Physics X, XX (2018), DOI: 10.1039/C8CP01676C.
Publications as doctoral or post-doctoral researcher
- A. Papakondylis, E. Miliordos and A. Mavridis, “Carbonyl boron and related systems: An ab initio study of B-X and YBBY (1Σg+), where X = He, Ne, Ar, Kr, CO, CS, N2 and Y = Ar, Kr, CO, CS, N2”, Journal Physical Chemistry A 108, 4335 (2004), DOI: 10.1021/jp031308q.
- E. Miliordos and A. Mavridis, “The electronic structure of vanadium oxide. Neutral and charged species, VO0,±”, Journal of Physical Chemistry A 111, 1953 (2007), DOI: 10.1021/jp067451b.
- E. Miliordos, A. Papakondylis, A. A. Tsekouras and A. Mavridis, “All-electron first principles calculations of the ground and some low-lying excited states of BaI”, Journal Physical Chemistry A 111, 10002 (2007), DOI: 10.1021/jp0745788.
- E. Miliordos and A. Mavridis, “Ab initio investigation of the electronic structure and bonding of BH, BH‾, and HBBH molecules”, Journal of Chemical Physics 128, 144308 (2008), DOI: 10.1063/1.2902284.
- E. Miliordos and A. Mavridis, “Theoretical study of the early 3d-transition metal diatomic oxides and their ions: ScO0,±, TiO0,±, CrO0,±, MnO0,± ”, Klaus Ruedenberg Special Issue (invited), Journal of Physical Chemistry A 114, 8536 (2010), DOI: 10.1021/jp910218u.
- E. Miliordos and A. Mavridis, “An accurate first principles study of the geometric and electronic structure of B2, B2¯, B3, B3¯, and B3H. Ground and excited states”, Journal of Chemical Physics 132, 164307 (2010), DOI: 10.1063/1.3389133.
- E. Miliordos, “Hückel versus Möbius aromaticity: The particle in a cylinder versus a Möbius strip”, Physical Review A 82, 062118 (2010), DOI: 10.1103/PhysRevA.82.062118. Highlighted in Science News issue of January 29, 2011 (page 16), https://www.sciencenews.org/article/twisted-rules-chemistry-explained.
- E. Miliordos and K. L. C. Hunt, “First principles calculations of the electronic and geometrical structures of neutral [Sc,O,H] molecules and the monocations, ScOH0,+ and HScO0,+”, Journal of Physical Chemistry A 115, 4436 (2011), DOI: 10.1021/jp110378d.
- E. Miliordos, “The particle in a Möbius wire and half-integer orbital angular momentum”, Physical Review A 83, 062107 (2011), DOI: 10.1103/PhysRevA.83.062107.
- C. N. Sakellaris, E. Miliordos and A. Mavridis, “First principles study of the ground and excited states of FeO, FeO+, and FeO‾ ”, Journal of Chemical Physics 134, 234308 (2011), DOI: 10.1063/1.3598529.
- E. Miliordos, J. F. Harrison and K. L. C. Hunt, “Ab initio investigation of titanium hydroxide isomers and their cations, TiOH0,+ and HTiO0,+ ”, Journal of Chemical Physics 135, 144111 (2011), DOI: 10.1063/1.3644963.
- X. Li, A. Mandal, E. Miliordos and K. L. C. Hunt, “Interaction-induced dipoles of hydrogen molecules colliding with helium atoms: A new ab initio dipole surface for high-temperature applications”, Journal of Chemical Physics 136, 044320 (2012), DOI: 10.1063/1.3676406.
- E. Miliordos, J. F. Harrison and K. L. C. Hunt, “Ground and excited states of vanadium hydroxide isomers and their cations, VOH0,+ and HVO0,+ ”, Journal of Chemical Physics 138, 114305 (2013), DOI: 10.1063/1.4793744.
- E. Miliordos and J. F. Harrison, “Hirshfeld density partitioning technique: a first application on transition metal compounds, TiO, VO, ScOH”, Journal of Chemical Physics 138, 184305 (2013), DOI: 10.1063/1.4803478.
- E. Miliordos, K. Ruedenberg and S. S. Xantheas, “Unusual inorganic biradicals: A Theoretical Analysis”, Communication to the Editor, Angewandte Chemie International Edition 52, 5736 (2013), DOI: 10.1002/anie.201300654.
- E. Miliordos and S. S. Xantheas, “Efficient procedure for the numerical calculation of harmonic vibrational frequencies based on internal coordinates” Joel M. Bowman Special Issue (invited), Journal of Physical Chemistry A 117, 7019 (2013), DOI: 10.1021/jp3127576.
- E. Miliordos, E. Aprà and S. S. Xantheas, “Optimal geometries and harmonic vibrational frequencies of the global minima of water clusters (H2O)n, n=2-6, and several hexamer local minima at the CCSD(T) level of theory” Journal of Chemical Physics 139, 114302 (2013), DOI: 10.1063/1.4820448.
- E. Miliordos and S. S. Xantheas, “Elucidating the mechanism behind the stabilization of multi-charged metal cations in water: A case study of the electronic states of microhydrated Mg2+, Ca2+ and Al3+”, Hot article for the week Oct 22, 2013 (http://blogs.rsc.org/cp/2013/10/22/this-weeks-hot-articles-11/), Highlighted in NERSC’s web page, June 2014, http://www.nersc.gov/news-publications/news/science-news/2014/thirsty-metals-key-to-longer-battery-lifetimes/. Highlighted in DOE’s Pulse (Science and Technology Highlights from the DOE National Laboratories, #417, 7 July 2014 http://web.ornl.gov/info/news/pulse/no417/story1.shtml. Reported in Science Springs, July 7 2014 http://sciencesprings.wordpress.com/2014/07/07/from-doe-pulse-satisfying-metals-thirst-vital-for-high-capacity-batteries, Communication to the Editor, Physical Chemistry Chemical Physics 16, 6886 (2014), DOI: 10.1039/c3cp53636j. Journal cover.
- E. Miliordos and S. S. Xantheas, “Unimolecular and hydrolysis channels for the detachment of water from microsolvated alkaline earth dication (Mg2+, Ca2+, Sr2+, Ba2+) clusters”, Thom H. Dunning Jr. Special Issue (invited), Theoretical Chemistry Accounts 133, 1450 (2014), DOI: 10.1007/s00214-014-1450-4.
- E. Miliordos and S. S. Xantheas, “On the bonding nature of ozone (O3) and its sulfur-substituted analogues, SO2, OS2, and S3: Correlation between their biradical character and molecular properties” Journal of the American Chemical Society 136, 2808 (2014), DOI: 10.1021/ja410726u.
- E. Miliordos, E. Aprà and S. S. Xantheas, “Benchmark Theoretical Study of the π−π Binding Energy in the Benzene Dimer” Journal of Physical Chemistry A 118, 7568 (2014), DOI: 10.1021/jp5024235.
- N. Sahu, S. R. Gadre, A. Rakshit, P. Bandyopadhyay, E. Miliordos and S. S. Xantheas, “Low energy isomers of (H2O)25 from a hierarchical method based on Monte Carlo temperature basin paving and molecular tailoring approaches benchmarked by MP2 calculations” Journal of Chemical Physics 141, 164304 (2014), DOI: 10.1063/1.4897535.
- J. C. Werhahn, E. Miliordos and S. S. Xantheas, “A new variation of the Buckingham exponential-6 potential with a tunable, singularity-free short-range repulsion and an adjustable long-range attraction” Chemical Physics Letters 619, 153 (2015), DOI: 10.1016/j.cplett.2014.11.051.
- T. Karman, E. Miliordos, K. L. C. Hunt, G. C. Groenenboom and Ad van der Avoird, “Quantum mechanical calculation of the collision-induced absorption spectra of N2–N2 with anisotropic interactions” Journal of Chemical Physics 142, 084306 (2015), DOI: 10.1063/1.4907917.
- E. Miliordos and S. S. Xantheas, “On the validity of the basis set superposition error and complete basis set limit extrapolations for the binding energy of the formic acid dimer”, Journal of Chemical Physics 142, 094311 (2015), DOI: 10.1063/1.4913766.
- E. Miliordos and S. S. Xantheas, “Ground and excited states of the [Fe(H2O)6]2+ and [Fe(H2O)6]3+ clusters: Insight into the electronic structure of the [Fe(H2O)6]2+–[Fe(H2O)6]3+”, J. Chem. Theory Comput. 11, 1549 (2015), DOI: 10.1021/ct501143c.
- E. Miliordos and S. S. Xantheas, “An accurate and efficient computational protocol for obtaining the complete basis set limits of the binding energies of water clusters at the MP2 and CCSD(T) levels of theory: Application to (H2O)m, m = 2-6, 8, 11, 16, and 17”, J. Chem. Phys. 142, 234303 (2015), DOI: 10.1063/1.4922262.
- E. Miliordos and S. S. Xantheas, “The origin of the reactivity of the Criegee intermediate: implications for atmospheric particle growth”, Angew. Chemie Int. Ed. 55, 1015 (2016), DOI: 10.1002/anie.201509685.
- C. T. Wolke, J. A. Fournier, E. Miliordos, S. M. Kathmann, S. S. Xantheas, and M. A. Johnson, “Isotopomer-selective spectra of a single intact H2O molecule in the Cs+(D2O)5H2O isotpologue: Going beyond pattern recognition to harvest the structural information encoded in vibrational spectra”, J. Chem. Phys. 144, 074305 (2016), DOI: 10.1063/1.4941285.
- E. Miliordos, E. Aprà and S. S. Xantheas, “A new, dispersion-driven intermolecular arrangement for the benzene-water octamer complex: Isomers and analysis of their vibrational spectra”, J. Chem. Theory Comput. 12, 4004 (2016), DOI: 10.1021/acs.jctc.6b00668.
- E. Miliordos, S. Caratzoulas and D. G. Vlachos, “A periodic-DFT study of retro-aldol fragmentation of fructose on MoO3”, Appl. Catal. A 530, 75 (2017), DOI: 10.1016/j.apcata.2016.11.021.
- T. B. Ward, E. Miliordos, P. D. Carnegie, S. S. Xantheas, and M. A. Duncan, “Ortho-para interconversion in cation-water complexes: The case of V+(H2O) and Nb+(H2O) clusters”, Journal of Chemical Physics 146, 224305 (2017), DOI: 10.1063/1.4984826.
Last updated: 09/08/2023