Research
Overview:
Research in the Goldsmith Lab focuses on using coordination compounds to promote redox activity. One overarching principle in the work is the use of ligand design to control the sorts of redox processes that proceed. Currently, there are three active projects: 1) magnetic resonance imaging (MRI) contrast agent sensors for reactive oxygen species, 2) catalysts for superoxide degradation, and 3) gallium- and cobalt-containing catalysts for the oxidation of organic molecules. Previously, we have investigated non-heme iron compounds as catalysts for the oxidation of C-H bonds by hydrogen peroxide and dioxygen. We collaborate extensively with researchers within and outside of Auburn University.
MRI Contrast Agent Sensors for Reactive Oxygen Species:
Reactive oxygen species, such as O2- anions, hydrogen peroxide molecules, and hydroxyl radicals, are intricately involved in many biological processes. The body carefully controls their concentrations, for excess amounts of these oxidants have been correlated to a wide array of inflammatory, cardiovascular, and neurological disorders (e.g. Parkinson's, Alzheimer's Diseases). The exact roles that reactive oxygen species play in pathology are still unclear. Current methodology for monitoring and assessing oxidative stress relies on either indirect methods, such as analyzing protein and lipid oxidation, and/or invasive tissue sampling. The development of sensors that can directly assess oxidative activity within whole-body subjects could allow us to better understand the connections between reactive oxygen species and disease and potentially allow the earlier diagnosis and treatment of several lethal and debilitating disorders. The primary goal of the project is to develop MRI contrast agents that exhibit changes in their relaxivity upon reaction with a reactive oxygen species. Commonly available MRI instrumentation can detect such changes within thicker biological samples without the necessity of invasive measures. Recent publications from the Goldsmith Group have reported manganese(II) complexes with redox-active ligands that exhibit changes in their T1-derived relaxivity upon reaction with H2O2. The changes in the relaxivity allows the manganese(II) complexes to act as sensors for H2O2. Notably, the responses do not require a co-analyte and display no response to O2. This work is being done in collaboration with the Auburn University MRI Research Center and the Auburn University College of Veterinary Medicine, was previously supported by a grant from AURIC, and is currently supported by the NSF. The below figure appears in a manuscript published in the Journal of the American Chemical Society, with two subsequent reports appearing in Inorganic Chemistry, including one that was a featured article. The project was recently highlighted by Auburn's College of Science and Mathematics.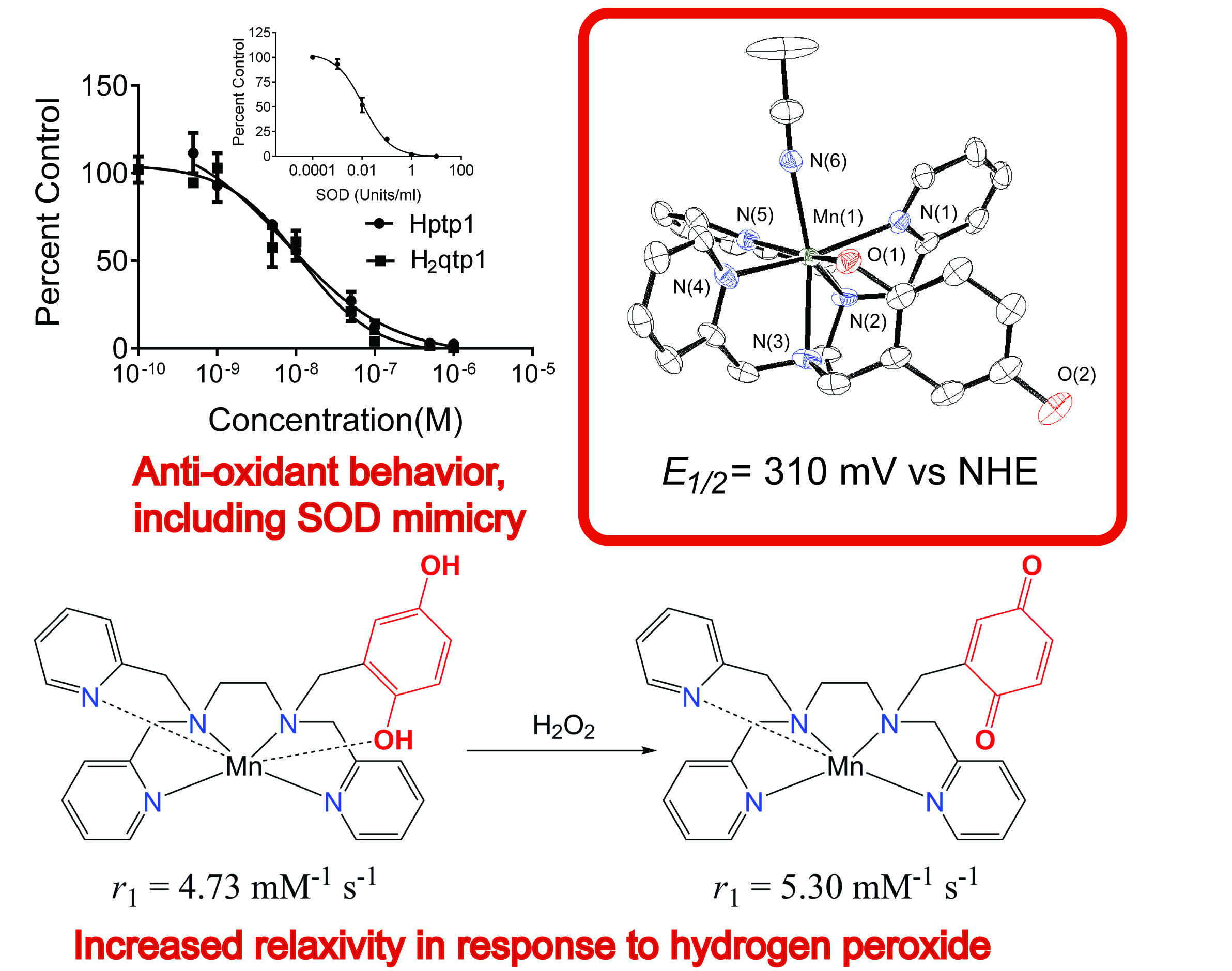
Catalysts for Superoxide Degradation:
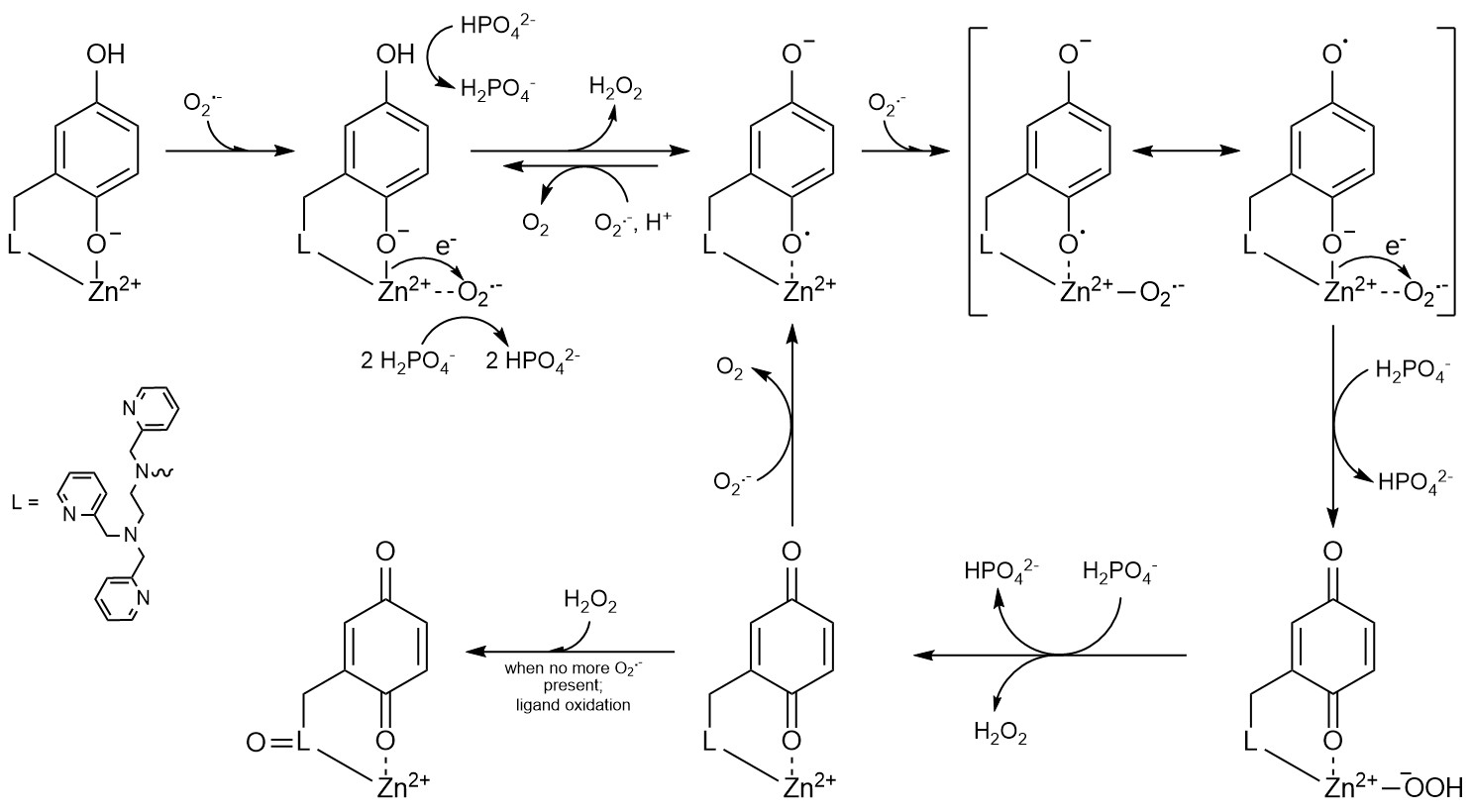
Given the likely involvement of reactive oxygen species in pathology, there exists considerable interest in developing antioxidants to control their concentrations. We found that the previously described MRI sensors display strong antioxidant activity, and these or related molecules may be useful for simultaneously imaging and treat oxidative stress in vivo. A study on the antioxidant properties of one of our sensors appeared in the Journal of the American Chemical Society; this work was done in collaboration with Prof. Ivana Ivanović-Burmazović from the Ludwig Maximilian University of Munich. More recently, we found that the redox-active ligands used in our more recent sensors could serve as redox partners for superoxide. Although the free organic molecules by themselves cannot catalyze superoxide degradation, a zinc(II) complex with one of the ligands can. This work, which appeared in Nature Chemistry, was the first instance of a coordination complex between a redox-inactive metal ion and a redox-active ligand mimicking the activity of superoxide dismutase. We are currently investigating how the choice of metal ion influences the antioxidant activity of coordination complexes with polydentate quinol-containing ligands.
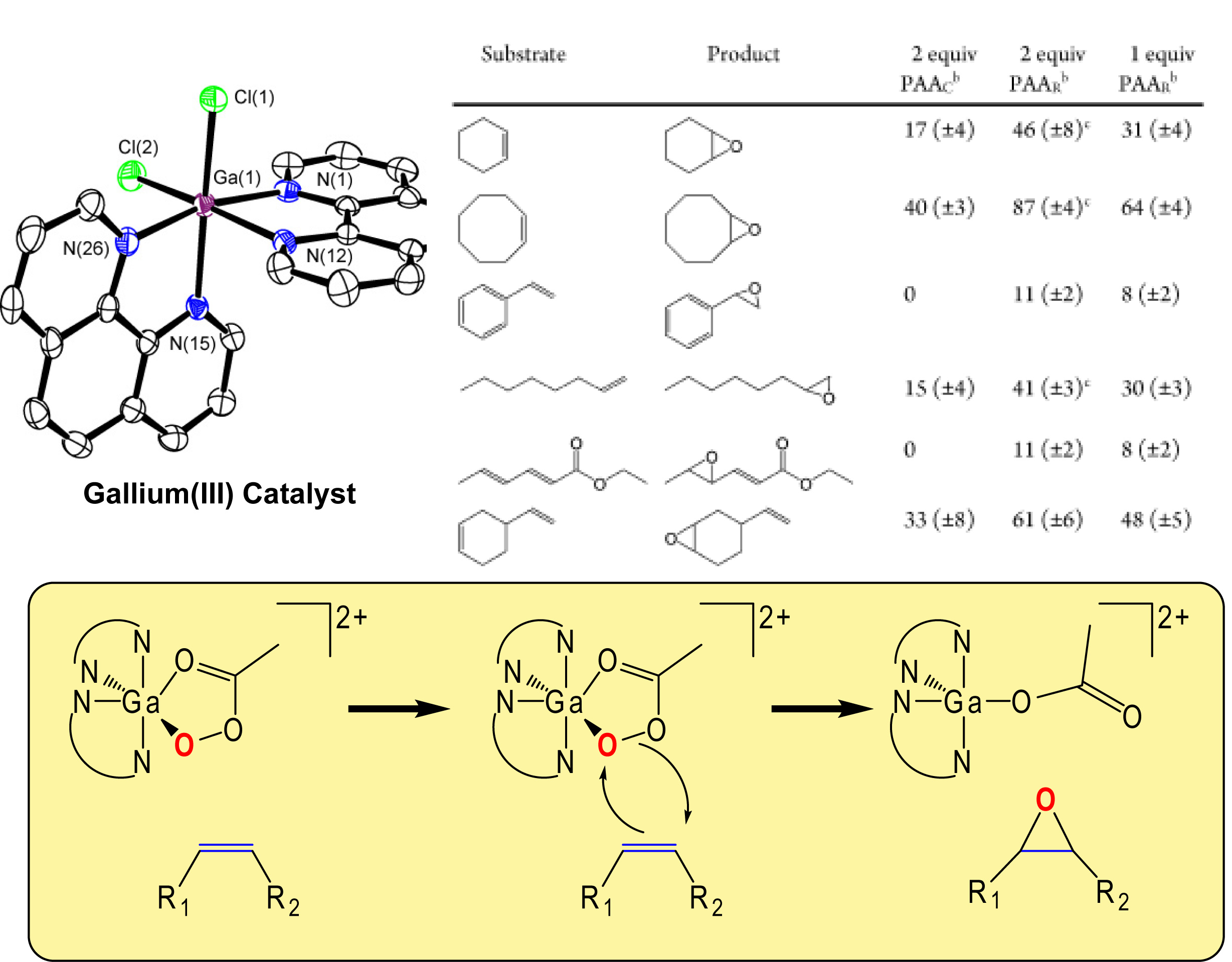
Gallium(III) Catalysts for Oxidation Reactions:
Gallium and other Group 13 metals are widely considered to be redox-inactive; the overwhelming majority of gallium compounds have the metal in the +3 oxidation state. Consequently, gallium compounds have not found wide application in the catalysis of reduction-oxidation reactions. Recently, we have investigated Ga(III) complexes with neutral N-donor ligands and have found them to be competent catalysts for the oxidation of alkenes to epoxides by peracetic acid. These represent the first instances of Ga(III) complexes serving as homogeneous catalysts for this class of reaction. The chemistry is astonishingly selective for the epoxide product and proceeds at room temperature or below in both organic and aqueous solvents. We currently speculate that the metal activates the terminal oxidant through a Sharpless-type mechanism. We have recently explored this mechanism in collaboration with Prof. Michael McKee. We have found similar activity for Al(III) complexes prepared by Prof. Christopher Graves and his research group at Swarthmore College. This work and similar research using cobalt have been supported by a grant from the American Chemical Society -Petroleum Research Fund.
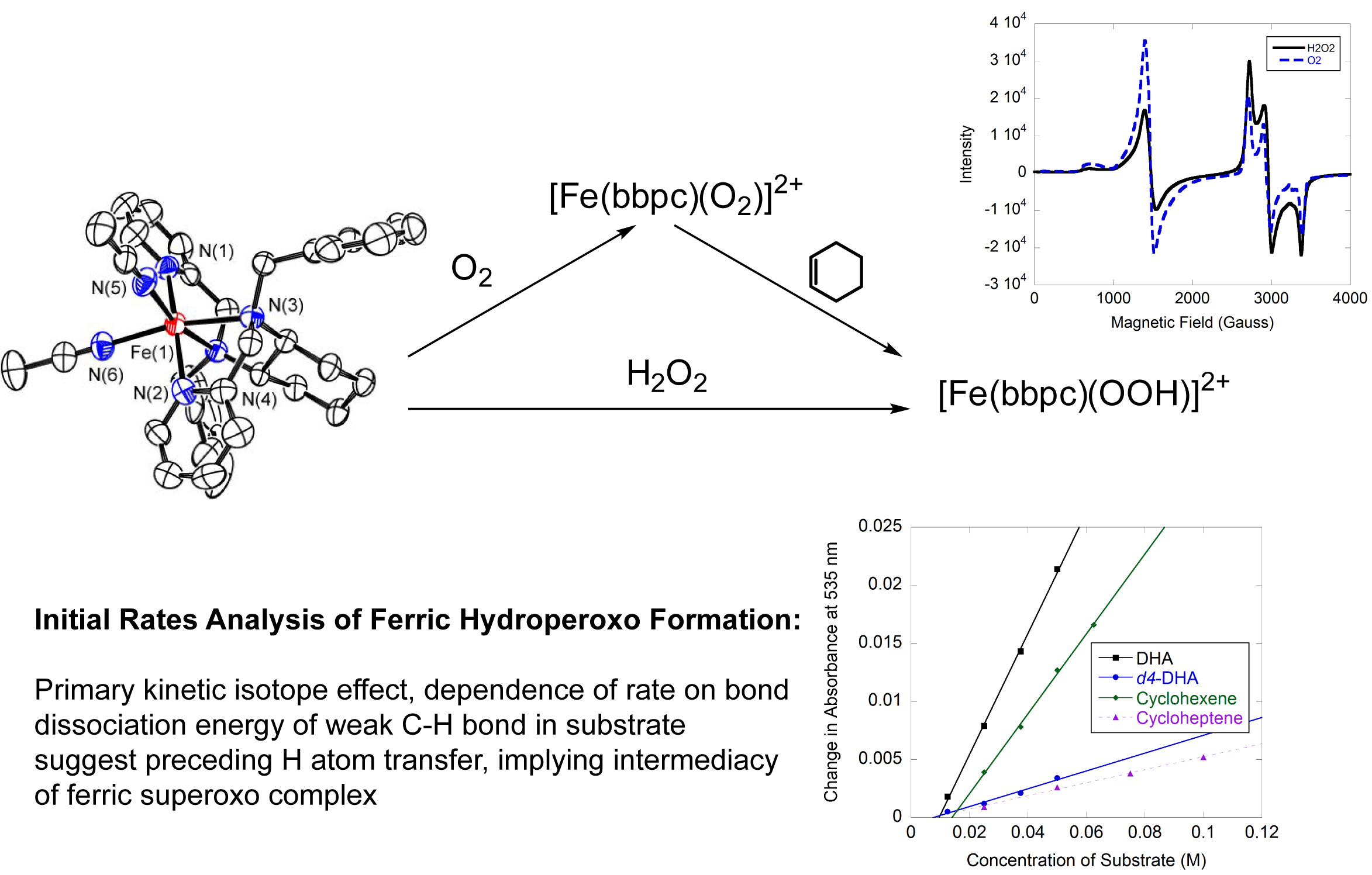
Non-Heme Iron Catalysis:
Non-heme iron enzymes known as hydroxylases catalyze the conversion of C-H bonds to C-OH functional groups using O2 as the terminal oxidant. The reactivity is of particular interest since it activates aliphatic C-H bonds, which are normally chemically inert. An additional attractive feature is that the catalyzed oxidation chemistry is regioselective, in that only certain carbon atoms within the substrate are oxidized. One former project within the Goldsmith Lab was to develop coordination compounds that could replicate this activity and direct the oxidation towards particular C-H bonds within either a single substrate or a group of substrates. We successfully used steric repulsions between the catalyst and the substrate to direct the catalyzed oxidation towards less sterically congested C-H bonds within substrate molecules. The second goal was to use O2 as the terminal oxidant instead of H2O2, which can be expensive to produce and difficult to transport. We successfully used a non-heme iron complex to catalyze the oxidation of hydrocarbons with tertiary aliphatic C-H bonds by O2 without the necessity of a sacrificial reductant. Further, we found compelling kinetic evidence for a ferric superoxo complex as the initial metal-based oxidant. In order to better characterize the compounds and the reactivity, we collaborated with Prof. David Stanbury and Prof. Evert Duin at Auburn University for stopped-flow kinetics and EPR analysis, respectively. The Goldsmith group is currently exploring other transition metals as catalysts for C-H activation. We recently found that a cobalt complex with a sterically encumbered ligand could catalyze the oxidation of weak C-H bonds by iodosobenzene.