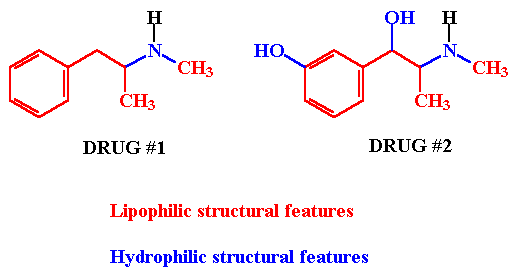
Examination of the structure of the following drug structures indicates the presence of both lipophilic (nonpolar) and hydrophilic (polar) groups and substituents in each structure. The relative solubility of each of these drugs is a function of the contributions of each of the individual structural features of these molecules. For example, the relative solubility of an OMA is the sum of the contributions of each group and substituent to overall solubility.

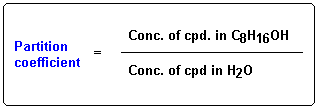
The relative solubility of organic drug molecules is measured by determining the extent of their distribution into an aqueous solvent (usually pH 7.4 buffer to mimic the physiological environment) and a lipid solvent (usually n-octanol to mimic the physiologic lipid environment). These experiments generate a value, P, known as the partition coefficient for each compound.
1. Comparison of Drug Structural Features
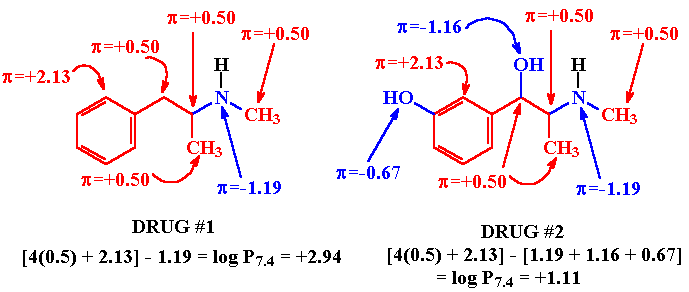
Given 2 or more drug structures, one should be able to estimate the relative solubility of each. For example, Drug #2 (above) is similar in structure to Drug #1 EXCEPT that Drug #2 contains two additional structural features - a phenolic OH function and a 2o-OH alcohol function. Since OH functions are considered to be hydrophilic structural features (they H-bond to solvent H2O molecules thereby increasing solubility in this medium) it would be reasonable to assume that Drug #2 is more hydrophilic than Drug #1 or, saying it another way, Drug #1 is more lipophilic than Drug #2.
2. Mathematical Estimation of Relative Solubility
Experimentation has shown that the relative solubility of drug structure can be estimated by summing the solubility contributions of individual groups and substituents in a drug structure. Quantitative values have been determined to express the various solubility contributions of the structural features of drugs. These solubility contributions are expressed as hydrophilic (negative) and lipophilic (positive) hydrophobic bonding constants.
A tabulation of some of these hydrophobic bonding constants is provided in Dr. Riley's slides on Drug Absorption.To calculate a log P for an OMA (i) the molecule is dissected into its various groups, functionalities and substitutents, (ii) appropriate hydrophobic bonding constants are assigned and summed and (iii) compounds with log Pcalc values greater than 0 are considered lipophilic molecules that will have solubility of less than 3.3% in water) and those with log Pcalc values less than 0 are considered to be hydrophilic compounds with higher H2O solubility.