David Stanbury David StanburyProfessor Inorganic Phone: (334) 844-6988 Email: stanbury@auburn.edu |
U. Southern California, Ph. D., 1978 Inorganic Chemistry: Mechanisms of inorganic redox reactions in solution.Dr. Stanbury's research is concerned with the mechanisms of inorganic redox reactions in solution. Redox reactions of main-group species such as I–, NH2OH, and SCN– are a special focus. Because of their ubiquity, these compounds engage in many real-world redox reactions. For example, oxidation of I– is essential to the operation of a large class of solar energy cells, oxidation of NH2OH is a central step in the global nitrogen cycle, and oxidation of SCN– is one of the major functions of human peroxidases. Study of these reactions also affords fine opportunities to develop fundamental insight into chemical reactivity, probing questions such as whether the solvent can act as a proton acceptor in so-called PCET (proton-coupled electron-transfer) reactions. One area of long-standing interest has been free-radical reactions. A typical example is the reaction of SCN– with [Ni(tacn)2]3+. The two tacn ligands bind the Ni(III) tightly and provide no sites for attack by SCN–. The Ni(III) complex is reduced to [Ni(tacn)2]2+ through an outer-sphere electron-transfer mechanism, and SCN– is oxidized to a free radical: SCN. Subsequent steps of the mechanism lead to its conversion to SO42–. The first step is endothermic: its microscopic reverse is exothermic, and is diffusion controlled. As a consequence, the barrier in the rate-limiting step is determined strictly by the thermodynamics of that step. Radicals such as NO2, NH2OH+, and ClO2 react with rates much slower than imposed by the effects of diffusion; their low reactivity seems to arise from the structural changes that they undergo as they accept electrons. An example of redox without electron transfer is the oxidation of NO by O2, which goes by way of atom transfer to form NO2. Another example is the disproportionation of N2H2; this has a pericyclic transition state with concerted transfer of two hydrogen atoms. In yet another example, NO is oxidized by Ni(III)L via attack of NO at a lone pair on the ligand L. Transformations such as these bypass the high-energy steps associated with outer-sphere electron transfer.
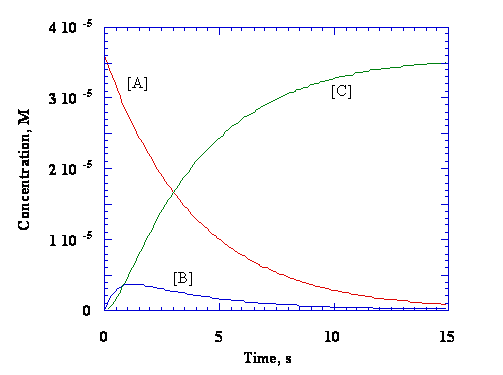
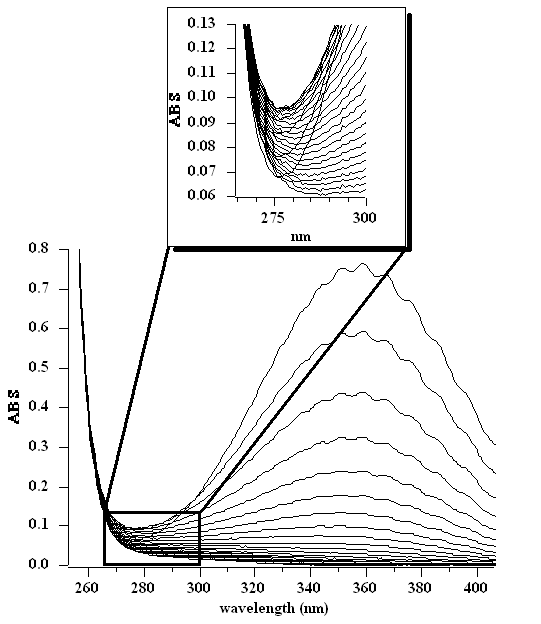
Multi-wavelength decay profile of the reaction of ClO2 and SCN–. The multiwavelength kinetic data were taken at 25 °C in 1 M HClO4, [ClO2]o = 0.37 mM, [SCN–]o = 25 mM. The full scan depicts spectra sparsed at 5.95 s intervals. The enhanced area shows only the first 100 s of the reaction at 3.96 s intervals, indicating that the reaction does not pass through an isobestic point. Bray, T. H.; Ling, J.; Choi, E.-S.; Brooks, J. S.; Beitz, J. V.; Sykora, R. E.; Haire, R. G.; Stanbury, D. M.; Albrecht-Schmitt, T. E. “Critical Role of Water Content in the Formation and Reactivity of Uranium, Neptunium, and Plutonium Iodates Under Hydrothermal Conditions: Implications for the Oxidative Dissolution of Spent Nuclear Fuel” Inorg. Chem. 2007, 46, 3663-3668. Wang, X.; Stanbury, D. M. “Direct Oxidation of L-Cysteine by [FeIII(bpy)2(CN)2]+ and [FeIII(bpy)(CN)4]–” Inorg. Chem. 2008, 47, 1224-1236. Song, Na; Stanbury, D. M. “Proton-Coupled Electron-Transfer Oxidation of Phenols by Hexachloroiridate(IV)” Inorg. Chem. 2008, 47, 11458-11460. Stanbury, D. M. “Reactivity of Inorganic Radicals in Aqueous Solution” in Physical Inorganic Chemistry: Reactions, Processes, and Applications, Bakac, A., Ed., John Wiley and Sons, NY, 2010, 395-428. Koppenol, W. H.; Stanbury, D. M.; Bounds, P. “Electrode potentials of partially reduced oxygen species, from dioxygen to water” Free Rad. Biol. Med., 2010, 49, 317-322. |
Last updated: 01/05/2012