COSAM News Articles 2023 May New study unravels secrets of reovirus binding mechanisms, offering hope for improved oncolytic therapies
New study unravels secrets of reovirus binding mechanisms, offering hope for improved oncolytic therapies
In a groundbreaking study recently published in PNAS, scientists have made significant progress in understanding the mechanisms of reovirus attachment to host cells. Reoviruses are known to trigger celiac disease development and possess oncolytic properties, making them a valuable tool in cancer therapy. A deeper comprehension of these mechanisms could potentially improve the effectiveness of oncolytic therapies.
Reoviruses are nonenveloped, icosahedral viruses containing a segmented, double-stranded RNA genome. While not commonly associated with human diseases, reoviruses have been found to disrupt the development of immunological tolerance to orally ingested gluten, leading to celiac disease. Additionally, reoviruses display oncolytic activity across various tumor types, showing promise for cancer treatment.
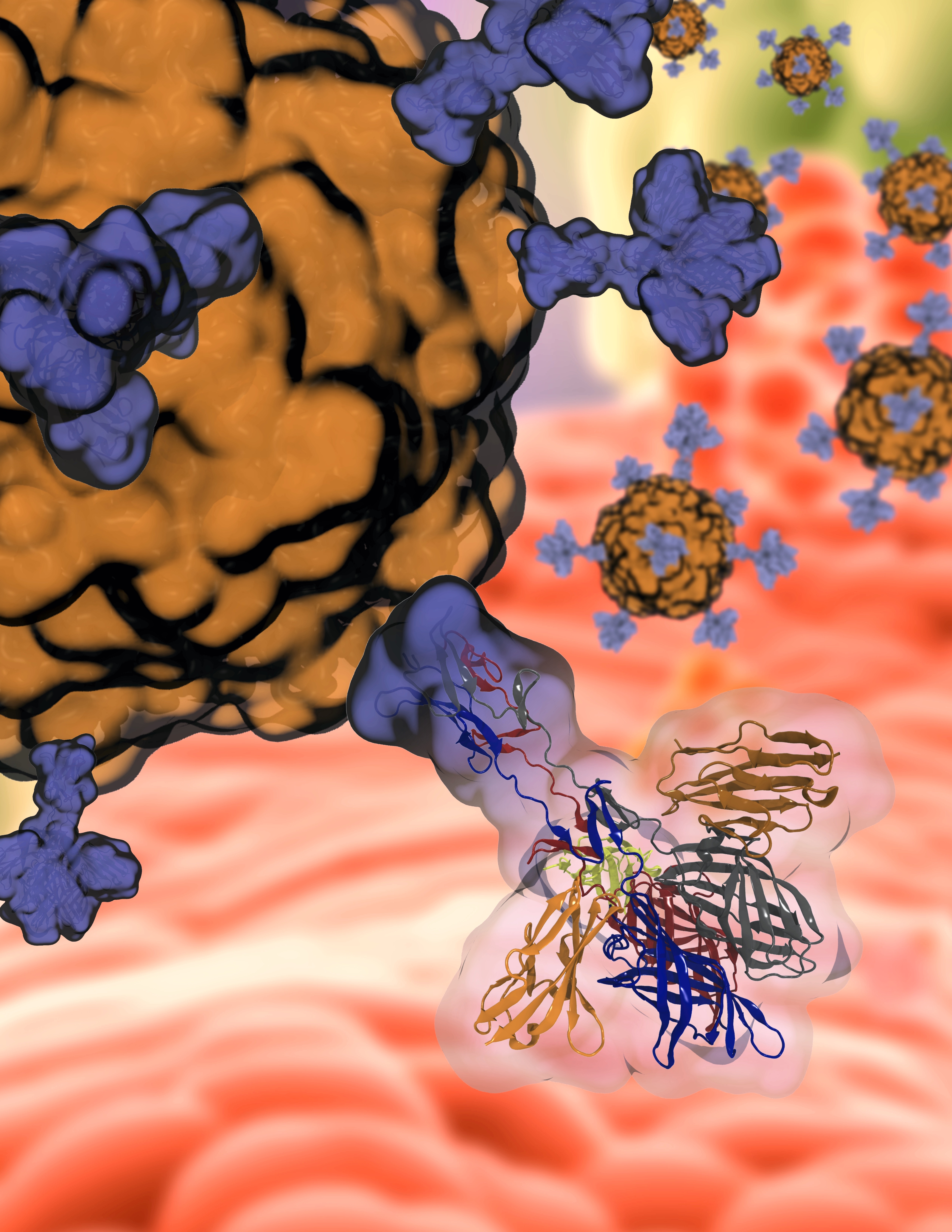
Rafael C. Bernardi, a biophysicist at Auburn’s College of Sciences and Mathematics says that “the initial attachment of viruses to cell surface receptors is a crucial determinant of a successful infection.” However, the dynamics and cooperativity between multiple cell surface receptors are not yet fully understood. In this study, in an effort that combined scientists in Belgium, Germany and the United States, the researchers combined force distance-based atomic force microscopy (AFM), single-particle tracking, and steered molecular dynamics (SMD) to investigate the role of conformational flexibility in the binding properties of the viral capsid protein σ1.
The study's findings suggest that reoviruses employ two distinct strategies for successful infection. First, sialic acids serve as molecular bridges between the virus and the receptor, and second, reoviruses adopt more extended protein conformers, both contributing to a more stable molecular complex and favoring virus entry.
These new insights into the mechanisms of viral attachment to cell receptors could pave the way for the development of more effective antiviral drugs and oncolytic vectors. By controlling the crosslinking of viral capsid proteins involved in binding to cell entry receptors, researchers could potentially improve specific cell targeting in the development of new oncolytic therapies.
Priscila Gomes, a distinguished postdoctoral researcher at Auburn's Department of Physics, emphasizes that "the pivotal computational work conducted at Auburn was crucial in unveiling the role of the sialic acids on the binding mechanism of Reovirus to its human host. The high-resolution of our simulations enabled us to explain the mechanism behind the host receptor affinity.”
Overall, this study sheds light on the intricate dynamics and cooperativity of reovirus interactions with host cell surfaces, providing a better understanding of the early binding stages crucial for infection. As scientists continue to unravel the secrets of reovirus binding mechanisms, they open new avenues for improving cancer treatment and other therapeutic applications.
Latest Headlines
-
04/23/2024
-
04/18/2024
-
04/18/2024
-
04/18/2024
-
04/17/2024