Icy Inverts Cruise 2013 - Shipboard Blog - Jan 22nd to Jan 25th

25 January 2013: within the Western Amundsen Sea (73° 39.7’S, 129° 23.1’W)
We successfully entered a polynia in the Western Amundsen Sea known as the Wrights Gulf a little over a day ago and have been intensely sampling ever since. Already we have conducted 3 plankton tows, 4 Mega-Core casts, 4 Blake trawls, and 2 mud grabs, to name a few. Sampling is still continuing as we begin to make our way back to the initial entry point of the polynia. As seen in previous blogs over the last couple of days, the area we are currently in has never been surveyed before, hence we are trying to collect as much as possible so we can make an accurate description of the area and the organisms that inhabit it.
One thing we have noted in the Wrights Gulf is that the diversity of animals that we have been collecting seems greater here than in our previous sample location. The diversity of different taxa (or types of organisms) seems to be higher, as well as the diversity within each group. For example, we collected a diverse number of sea spiders (above picture) in one of our latest Blake trawls. In other locations we might see 2 different types of sea spiders, but in the Wrights Gulf, we have at least 6-7 different species.
We have also been impressed with the size of the organisms we are finding here. In another Blake trawl here we found a large Dumbo octopus as well as one of the largest scale worms most of us have seen. Several of us took the opportunity to have our photo with the worm (as Matt Galaska is seen doing in the below picture). As word trickled to those of us not on shift yet, we all rushed to take a moment to really examine these creatures. With every trawl, I am still amazed with animals we are collecting. The excitement of what we may find sometimes distracts several of us from getting much needed sleep.
Well, my shift is about to begin and the last Blake trawl was filled with a lot of mud, which takes a while to go through. I should get back downstairs and continue to help with the processing of the samples. Until next time…..
Contributed by: Dr. Pamela Brannock, Postdoctoral Fellow, Auburn University

***************************************************************************************

24 January 2013: Somewhere in the Western Amundsen Sea (73° 11.2’S, 129° 36.8’W)
I peeked out my bedroom porthole this morning but all I could see was white. There was no delineation between the uniform white ice and an overcast sky as far as the eye could see. It was like nothing else existed but the ship and a vast, bright emptiness – one giant blind spot. We’re currently breaking ice to get into a polynya in the Western Amundsen Sea. The ice is actually so thick in places that, at times, the ship actually has to stop, back up, and ram it again. If all goes well, hopefully we will make it into the polynya sometime this evening and start sampling shortly thereafter.
In our previous blog posts, we’ve talked a lot about how we are collecting specimens of invertebrates and what we will be doing with them (phylogeography, systematics, describing new species, etc). What we haven’t talked a lot about is how specimens are preserved. This may sound like a trivial issue, but it is actually a lot more complicated than you might think. Unfortunately, in our line of research, we have to sacrifice individual animals in order to study them. I want to emphasize that all of the specimens that we collect will end up in catalogued museum collections. Not even the smallest worm will die in vain.
When live organisms come up in the trawl, the first thing we do is get them back in sea water or at least keep them cold until they can be photographed. It’s very important to photograph living animals because they often change in color or shape after they die. Fortunately we have Dr. Christoph Held on board, who takes amazing photos of the animals. We have another camera set-up for “quick and dirty” photos of virtually every animal that comes up (above and below picture).

Next, we need to preserve specimens in different ways for different uses. Formalin is a fixative used to preserve specimens for studying their anatomy or morphology. Specimens preserved in formalin can be used for a technique called histology, where you make thin slices through an organism and look at them under a microscope to study anatomy. Unfortunately, it is very difficult to extract DNA from specimens preserved in formalin, so usually only one or a few voucher specimens are fixed in formalin and most of the rest are fixed in other ways. Specimens preserved in ethanol generally don’t look as nice as they would when preserved in formalin but it is easy to get DNA from them for molecular studies. The same is true for specimens preserved in a fixative called RNAlater that preserves both DNA and RNA. RNAlater is pretty expensive stuff so usually only a small tissue sample of each organism is preserved this way. Finally, a lot of our specimens also get wrapped up in aluminum foil or put into plastic bags and go into the -80°C freezer. Frozen specimens can be used for DNA and RNA extraction and in some cases they can even be used to study morphology. The only downside to freezing specimens is that there could be a real problem if a freezer breaks down or a box gets delayed in shipment. We generally avoid putting all of our eggs in one basket (below picture).
There is often a conundrum when we only collect one specimen of an organism. If we collect only one specimen of a really interesting worm, should it go into formalin so we can study its anatomy? Or should it be frozen or put in ethanol so we can study its genes? Fortunately, only a tiny piece of tissue is needed to get enough DNA for routine laboratory work. Usually we are able to have our cake and eat it too (gross analogy when I’m talking about worms, but you get my meaning).
As you can imagine, we have quickly accumulated a large collection of jars, tubes, and bags containing specimens. In the coming days, we’ll talk more about how we keep track of everything and how all of our specimens and gear will eventually make their way from McMurdo Station, Antarctica through New Zealand, and back to our home institutions.
Contributed by: Kevin Kocot, Ph.D. Candidate, Auburn University

***************************************************************************************

23 January 2013: Approaching the Wright Gulf (70° 14.5’S, 123°44.0’W)
The remoteness of where we are is staggering to me on a daily basis (example in above picture). It hits me when I try and send an email and can’t, simply because we are in a region where satellite coverage is sparse at best. We are about seven days away from other humans by boat if we headed straight to their location (McMurdo Station). If we were to travel due north from where we are right now, the first land would be somewhere around the Los Angeles, California coast. We truly are in the middle of nowhere…
This is my third research trip to Antarctica. I’ve been asked by a number of people why I keep coming back, considering the large number of potentially detracting variables that include the Drake Passage, being away from family, isolation, the cold, sea-sickness, the Drake Passage (yes, I put it there twice on purpose (below picture)…it can truly be that bad). Right now we are steaming away from the eastern portion of the Amundsen Sea where only our group and one other (the British Antarctic Survey) had ever been to collect benthic organisms. It was gorgeous. Along with the great science we got to complete, there were seals, whales, penguins, and some of the most beautiful icebergs I’ve ever seen. Our next destination is a polynia located in the western Amundsen Sea…. and an area that no scientific survey has ever been…our group will be the first!! That’s one of the major draws for me. We will be collecting samples from a place on planet Earth that no one has ever been sampling the benthic animals and the likelihood of people coming back is slim. Why do I keep doing this? Simple. I get to see things and go places that few, if any, humans have ever gone. Because of this, and because we get to do some amazing science to boot, I’ll keep coming back as long as I am fortunate enough to do so.
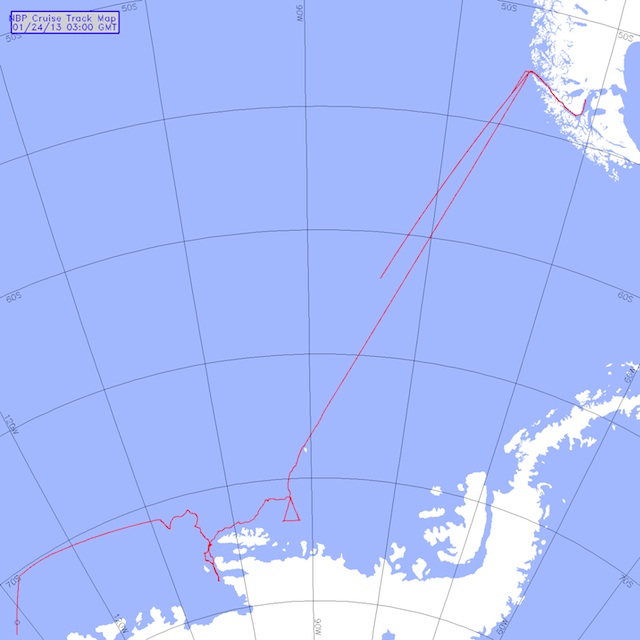
We are approaching this novel area by tracking the northern extent of the ice edge (above picture). The wind patterns have changed and we are not sure how easy it will be to get into this region known as Wrights Gulf. The continental shelf is close to shore in this area and we have to try and go through a thick band of ice (80+ miles). This takes time. In open ocean, we travel at about 10 knots, or 10 nautical miles an hour. Depending on ice conditions, our speed will drop to 6 or maybe 4 knots. If the ice is too thick, we need to back up the ship and literally ram the ice with it to break the ice up ….this means 1 or 2 knots. How slow is that, well you know how slow 10 miles an hour seems in a car, 10 knots is only slightly faster. For the last 200 miles, we have been in and out of foggy conditions. That can be dangerous because we are still around lots of large icebergs (below picture). Fortunately, the ships radar is good at picking them up and informs the ships mates what to avoid.
So it is a lot of work to get to such remote areas, but it is truly amazing in terms of the scenery and the thrill of being a one of the few to set eyes on it.
Contributed by: Dr. Andrew Mahon, Assistant Professor, Central Michigan University

***************************************************************************************
22 Jan 2013: North of the Sea Ice (70o 11.9`S, 121o 48.0`W)
Greetings to everyone following our adventure!
We’ve moved out of the ice and are back in open waters, transiting to our next sampling station. Being out of the ice means we’re making better time but it also means we are exposed to the ocean and winds. So we’re back to rocking and rolling. Makes it fun to walk around the ship (not really).
The main purpose of our voyage is to collect samples for researchers to examine various questions related to the benthic communities of the Antarctic continental shelf. As one of many students onboard, this trip has multiple purposes for us. The sampling we are doing provides me with some of the organisms I will need to examine the major questions that will be the focus of my Ph.D. dissertation. The sampling work itself provides real world field experience that will help me lead expeditions like this when I’m a full-fledged researcher. The time spent with other researchers and students from different organizations, expands my knowledge of questions being asked by others and of organisms that are not my focus. It can be easy to become preoccupied with your particular organism or question, so it’s good to see what others are doing. Seeing the animal of interest in its habitat also helps me to better understand it and hopefully frame better questions as I continue my research. All of this combines to make for an intense, rewarding educational experience that would be impossible to get in a classroom.
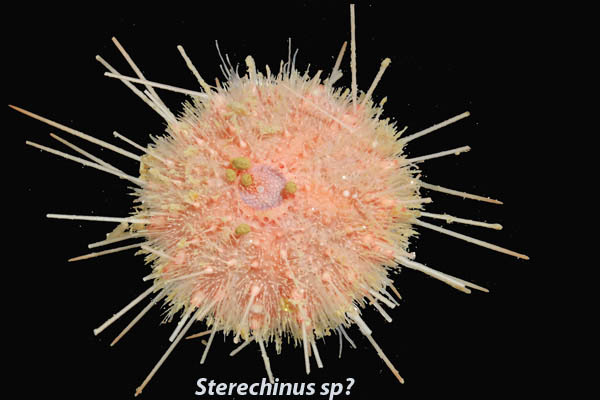
One of the more difficult things for me to pick up on this trip has been the scientific names of the various animals. Some of the people with me can rattle off the names of most things they see. Some of that comes from experience but it’s also a talent I think, sadly, I don’t seem to have. Animals generally have a common name like sea urchin or seastar or red headed woodpecker and a scientific name which is made up of two parts, the genus and the species. I am studying a particular sea urchin whose scientific name is Sterechinus neumayeri (similar to above picture). Sterechinus is the genus and neumayeri is the species. Animals that are very closely related will share the genus and have different species names. There is another urchin here called Sterechinus antarcticus. They are closely related but distinct species. They are very difficult to tell apart with the naked eye. We are also finding a substantial number of irregular urchins and they share the genus Abatus but species are A. curvidens and A. cordatus (below picture). While Sterechinus and Abatus are both urchins, they are less closely related and are a little further apart on the trees that Dr. Santagata described yesterday (Jan 21st blog). I’m getting better at learning the names but I constantly have to run through them in my head to get them to stick. Notice that the genus and species are italicized. That is the proper way to format scientific names in documents.
Live long and prosper,
Contributed by: David Branson , Ph.D. Candidate, Auburn University www.nullpilot.com

Last updated: 11/12/2013
