1. Draw skeletal structures of and give the IUPAC names for 9 compounds having the formula C4H8Br2 (18%).
2. Draw 3 dimensional structures of staggered and eclipsed (about the 1-2 bond) of 2,2-dimethylpropane. We have said that eclipsing methyl-hydrogen interactions raise the energy by 1.4 kcal/mol while eclipsing H-H interactions raise the energy by 1 kcal/mol. Given this information, estimate the energy difference between the structures you have drawn. Indicate which is more stable (18%).
3. Draw 3 dimensional structures of all conformers of both cis and trans
1-iodo-3-methylcyclohexane and answer the following questions (24%):
a. Indicate which structures correspond to the cis and the trans
b. Given that iodo is bigger than methyl, number the structures you have drawn in order of increasing stability (1 is the least stable)
c. Which is more stable cis or trans-1-iodo-3-methylcyclohexane? Justify your answer.
4. Light catalyzed reaction of chlorine gas with methane gas gives chloromethane. Write the mechanism for this reaction. Be sure to include all steps (12%).
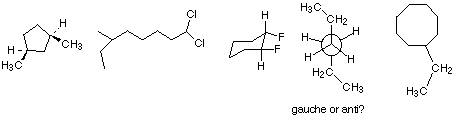
5. Name the following molecules (10%):

6. We have seen that given a heat of combustion of cyclohexane of 944.5 kcal/mol and a heat of combustion of cyclopropane of 499.8 kcal/mol we can estimate the strain energy of cyclopropane. In the space below, draw an energy diagram showing how this is done and estimate the strain energy of cyclopropane (18%).