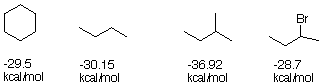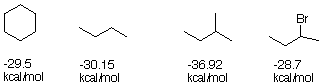CH504/628 Problem Set #1
Due 8 AM, 10/8/97
1. Given the following heats of formation, derive a set of group
equivalents which will allow you to calculate the heat of formation
of 1 bromo-2-methylcyclohexane.
2. Of course, your above analysis applies to both cis and trans
1-bromo 2-methylcyclohexane. Perhaps we can use molecular mechanics
(MM) to get information about each of the isomers and their
conformers.
a. Use MM to calculate the heat of formation of each conformer of
each of the above isomers.
b. Which is the most stable conformer of each isomer?
c. Which is the most stable isomer?
d. Examine the MM output and comment on the reasons for the
differences in stabilities.
e. Comment on the agreement between your estimated value from
problem 1 and the calculated values.
f. Is there anything in the calculation that surprised you?
3. Draw the 3 dimensional structure of the molecule whose
z-matrix is shown below:
1 C
2 C 1.34 1
3 C 1.34 120.0 2 1
4 O 1.41 109.5 0.0 3 2 1
5 H 1.09 109.5 -120.0 3 2 1
6 H 1.09 109.5 120.0 3 3 1
7 H 1.09 105.0 180.0 4 3 2
8 Cl 1.73 120.0 180.0 1 2 3
9 H 1.09 120.0 0.0 1 2 3
3. We often use heats of hydrogenation to estimate quantities
such as conjugation energy and strain energy. For example the heat of
hydrogenation of 1-butene is 30.3 kcal/mol (the heat of hyd = -32.8
kcal/mol) and that of 1,3-butadiene is 57.1 kcal/mol. These facts
have been used to estimate a conjugation energy of 3.5 kcal/mol for
1,3-butadiene.
a. Draw an energy diagram showing how this conjugation energy is
estimated. If you need help, consult an undergraduate organic
text.
b. Use MM to calculate these heats of hydrogenation and estimate the
conjugation energy. Note that the PCMODEL program allows you to
calculate with and without a pi correction. Do both and see which is
closer to the experimental value.
c. Calculate a barrier to rotation about the central bond in
1,3-butadiene with and without corrections. Discuss the relationship
between this barrier and the conjugation energy.
d. The heat of hydrogenation of 1,2-dimethylcyclopropene is 43.3
kcal/mol and that of 1-methylcyclobutene is 28.5 kcal/mol. Calculate
these values using MM and comment on the results.