MED. CHEM. II
Clinically Relevant Properties
of Organic Medicinal Agents
At the most fundamental level, the ability of a chemical compound to elicit a pharmacologic/therapeutic effect is related to the influence of various physical and chemical (physicochemical) properties of the chemical substance on the biomolecule(s) that it interacts with. Among the most pharmacologically influential physicochemical properties of organic medicinal agents (OMAs) are:
SOLUBILITY OF ORGANIC MEDICINAL AGENTS
Solubility characteristics of OMAs are of importance in regard to (1) formulation of the drug an appropriate dosage form and (2) its disposition in the living system (biodisposition) after administration.
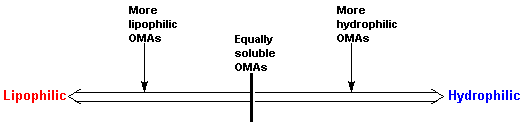
The solubility of an OMA may be expressed in terms of its affinity/philicity or repulsion/phobicity for either an aqueous (hydro) or lipid (lipo) solvent.
It is important to note that a majority of OMAs possess the property of balanced solubility, that is, most OMAs have some degree of solubility in both aqueous and lipid media. This is because there is a need for OMAs to move through both aqueous (plasma, extracellular fluid, cytoplasm, etc.) and lipid media (biologic membranes) in the biological system. Hence, solubility of OMAs should be viewed as being on a continuum between high lipophilicity on one end of the spectrum and high hydrophilicity on the other.

In order for a chemical compound to dissolve in a particular solvent/medium the compound must establish attractive forces between itself and molecules of the solvent. Hence, it is possible to estimate the solubility properties of an OMA (hydrophilic vs. lipophilic) by examining the structure of the OMA and noting whether its structural features promote affinity for aqueous or lipid media.
The most important intermolecular attractive forces (bonds) that are involved in the solubilization process are described as follows.
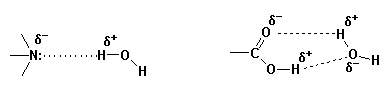

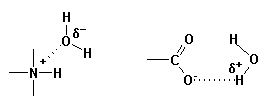
Hence, the relative solubility of an OMA is a function of the presence of both lipophilic and hydrophilic features within its structure which serve to determine the extent of interaction of the OMA with lipid and/or aqueous phases. A precise value for the relative solubility of an OMA can be determined in the laboratory, i.e. the partition coefficient. Also, procedures have been developed to estimate the relative solubility of an organic molecule based upon differential contributions of various structural features to overall solubility. Click here to access a primer on estimating the solubility of OMAs.
 Return to:
PY421 Home Page
Return to:
PY421 Home Page