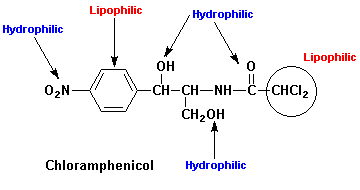
Examination of the structure of an OMA, e.g. chloramphenicol (see below, indicates the presence of both lipophilic (nonpolar) and hydrophilic (polar) groups and substituents. As noted previously, the physicochemical properties of an OMA arise from contributions of each of these structural features. For example, the relative solubility of an OMA is the sum of the contributions of each group and substituent to overall solubility.

The relative solubility of an organic compound is measured by determining the extent of its distribution into an aqueous solvent (usually pH 7.4 buffer) and a lipid solvent (usually n-octanol). These experiments generate a value, P, the partition coefficient for that particular compound.
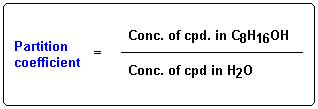
Experimentation has shown that the relative solubility of an OMA can be estimated by summing the solubility contributions of individual groups and substituents the OMA structure. Solubility contributions are expressed as hydrophilic (negative value) or lipophilic (positive value) fragment constants.


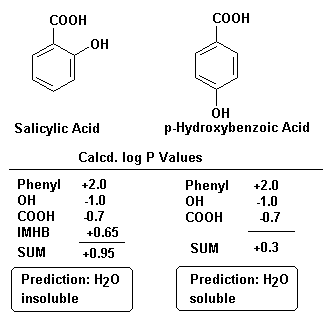
To calculate a log P for an OMA (i) the molecule is dissected into its various groups, functionalities and substitutents, (ii) appropriate hydrophilic/lipophilic fragment constants are assigned and summed and (iii) compounds with log Pcalc values greater than +0.5 are considered water insoluble (lipophilic, solubility is less than 3.3% in water) and those with log Pcalc values less than +0.5 are considered water soluble (hydrophilic).
Log Pcalc for chloramphenicol (see above) would be +2.1, a lipophilic compound [aromatic-NO2 = -0.28; three OH functions = -2.0; O=C-N function = -0.7; phenyl = +2.0; four C's = +2.0 and 2 Cl's = +1.0].
 Return to:
PY421 Home Page
Return to:
PY421 Home Page