Tectosilicates, Continued:
The Feldspathoids

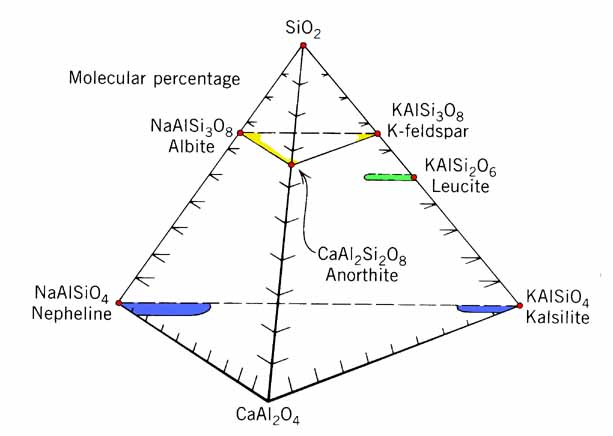
Feldspathoids, principally the minerals leucite, nepheline, kalsilite and sodalite, are anhydrous framework silicates similar to feldspars, but with lower SiO2 content. The feldspathoids tend to form from melts rich in alkali's and poor in silica. Although they are far less common than the feldspars, the unique settings in which feldspathoids occur are often of great tectonic and economic importance. Where present in sufficient quantity and purity, nepheline is mined for use in the manufacture of glass and porcelain.
| Leucite | KAlSi2O6 | Tetragonal (high-T)/ Isometric |
| Nepheline/ Kalsilite |
NaAlSiO4/ KAlSiO4 |
Hexagonal |
| Sodalite | Na8(AlSiO4)6Cl2 | Isometric |
| Lazurite | (Na,Ca)8(AlSiO4)6(SO4,S,Cl)2 | Isometric |
The feldspathoids have lower specific gravity (<2.5) than the feldspars (>2.5), and permit substitution of large cations, because of larger cavities in 4- and 6-member SiO4 rings. Large anions and anionic complex are housed in these cavities, and coordinated to oxygen in the enclosing rings.
Formulas for the feldspathoids may be derived simply by considering Ab-An solid solution, and taking out one SiO2 (for leucite), or Si2O4 (for nepheline). The sodalite formula is as six sodium nepheline, with 2 more Na and Cl to balance. Similarly, Lazurite is as six nepheline, but with Ca and sulfate in addition to Na and Cl.
| Na-Scapolite | 3NaAlSi3O8-(NaCl) | Tetragonal |
| Ca-Scapolite | 3CaAl2Si2O8-(CaSO4) 3CaAl2Si2O8-(CaCO3) |
Tetragonal |
Scapolites are tectosilicates with compositions suggestive of feldspars. The formulas are basically three time albite or anorthite, with a halite- or anhydrite-like complex.
Scapolite structures consist of corner-sharing Al and Si-tetrahedra that enclose large cavities. These cavities hold the anion complexes. There is complete solid solution in the scapolite series. Scapolite is a common and distinctive mineral in metamorphosed, siliceous limestones.
Zeolites
In a manner similar to the feldspars, feldspathoids, and scapolite, the zeolites are framework silicates with anions, anion complexes, and H2O housed structural voids. The hardness and specific gravity of the zeolites are among the lowest of the tectosilicates, reflecting their open structure. The voids in zeolite are actually interconnected spaces or channels, and thus zeolites have both porosity and permeability. In addition, zeolites can incorporate or loose water, and cations bound to (OH), as a function of temperature. In other words, heating and cooling zeolites causes them to loose or incorporate both volatile anions and certain cations. These properties, and fact that zeolites can now be commercially synthesized, results in uses of zeolites as 'molecular sieves' or absorbants in many household and industrial applications (everything from cat-litter to catalytic converters).
For other web-discussions and photos of zeolites, to supplement the text, try:
http://mineral.galleries.com/minerals/Silicate/Zeolites.htm
To check out some of the household applications, and see that there is a right zeolite for you, try:
A neat survey of zeolites, their uses, and cutting-edge research is provided in a 'cyber interview' between a highschool student and a NASA scientist: