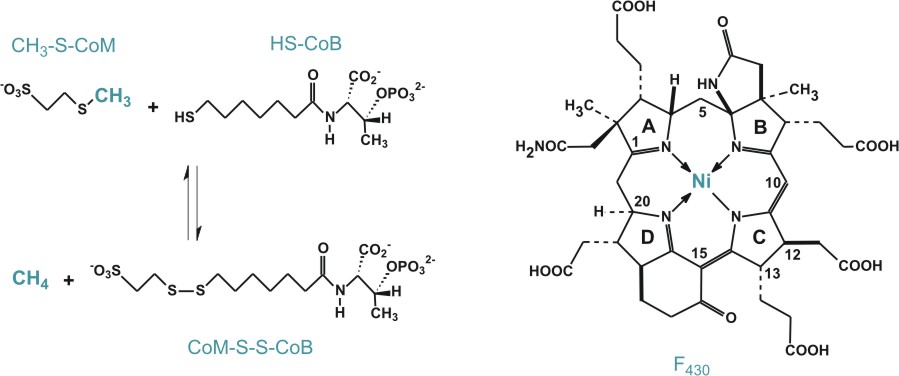
|
In nature,
there are two pathways found for the synthesis of the isoprene
precursors isopentenyl pyrophosphate (IPP) and dimethylallyl
pyrophosphate (DMAPP). These two compounds
are very important as the building blocks for the essential
biological molecules called isoprenoids, which include vitamins,
cholesterol, steroid hormones, carotenoids and quinones. Mammals,
including humans, use the mevalonate pathway to synthesize the
isoprene precursors, while eubacteria and some other microorganisms
use the DOXP/MEP pathway as the sole pathway for isoprene synthesis.
Several of the microorganisms that utilize the DOXP/MEP pathway are
pathogens, causing, for example, malaria, multidrug resistant
tuberculosis (MDR-TB), anthrax, plague, gastro-intestinal ulcers
and venereal diseases. This makes the DOXP/MEP pathway an attractive target for the development of new
anti-infective drugs. Since this pathway is not present in humans
these inhibitors should demonstrate very low toxicity.
Detailed knowledge of the mechanism and
regulation of the DOXP pathway is a prerequisite for the rational
design of inhibitors that are potential candidates for new
anti-infective drugs; however, due to its recent discovery the
function and catalytic mechanism of some of the proteins in this
pathway are not well understood. The goal of the proposed research
is to understand the reaction mechanism of the last two proteins in
the DOXP pathway, IspG ((E)-4-hydroxy-3-methylbut-2-enyl diphosphate
synthase; also known as GcpE) and IspH
((E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase; also known
as LytB).
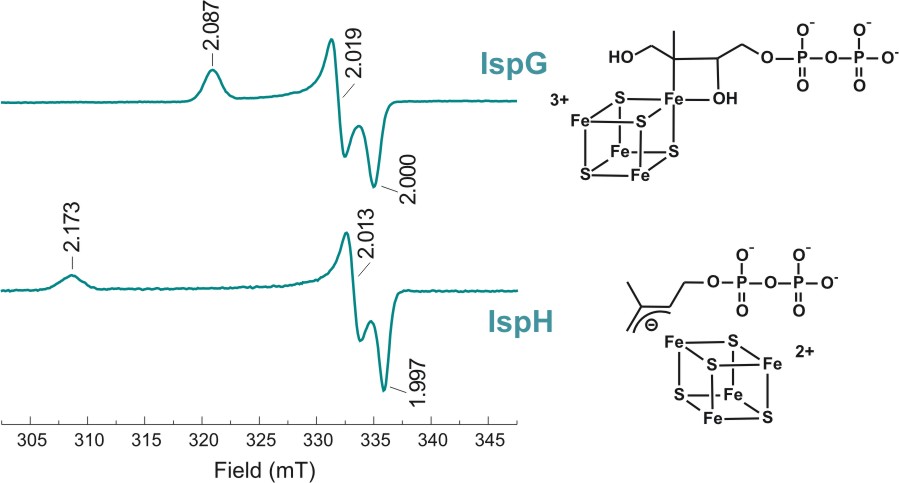
Both enzymes contain an iron-sulfur cluster in their
active site which appears to be involved in direct binding of the
respective substrates. Although both enzyme catalyze a similar step,
the reductive removal of a hydroxyl group, the proposed reaction
intermediates are very different. Our research is focused on
understanding these differences and to obtain a full understanding
of the different reaction steps in both mechanisms.
The synthesis part of the project is done in
collaboration with
Forrest Smith. Working on this project are
Selamawit Ghebreamlak
and Xiao Xiao
Papers:
-
Xu, W., Lees, N.S., Hall, D., Welideniya, D.,
Hoffman, B.M., Duin, E.C. (2012) A closer look at the
spectroscopic properties of possible reaction intermediates in
WT and mutant (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate
reductase (IspH/LytB). Biochemistry, 51, 4835−4849
-
Xu, W., Lees, N.S., Adedeji, D., Wiesner, J.,
Jomaa, H., Hoffman, B.M., Duin, E.C. (2010) Paramagnetic
intermediates of (E)-4-hydroxy-3-methylbut-2-enyl
diphosphate synthase (GcpE/IspG) under steady-state and
pre-steady-state conditions. J. Am. Chem. Soc., 132,
14509-14520
-
Rekittke, I., Wiesner, J., Röhrich, R., Demmer,
U., Warkentin, E., Xu, W., Troschke, K., Hintz, M., No, J.H.,
Duin, E.C., Oldfield, E., Jomaa, H., Ermler, U. (2008)
Structure of (E)-4-hydroxy-3-methyl-but-2-enyl
diphosphate reductase, the terminal enzyme of the non-mevalonate
pathway. J. Am. Chem. Soc., 130, 17206-17207
This project received funding from NSF. |