Our research area is theoretical and computational chemistry.
We employ state-of-the-art quantum chemical approaches to tackle important problems of modern chemistry
and explore new chemical phenomena. Currently, we work heavily on transition metal chemistry, f-block compounds, and solvated electron systems.
Our research is currently funded by NSF, DoE, and intramural AU grants. Our targeted topics are described below.
Solvated Electron Precursors (SEPs)
We recently discovered a novel class of molecules, called SEPs, which can bind electrons in their periphery.
These electrons occupy orbitals very similar to the atomic s,p,d,f-type orbitals.
SEPs are metal-ammonia complexes which they form a positively charged metal-ammonia core and one, two, or three electrons
have been found to orbit around it. Our first example is the Be(NH3)40,+,- species [DOI: 10.1021/acs.jpclett.7b03000].
Below we show some selected atomic-like orbitals of such outer electrons:
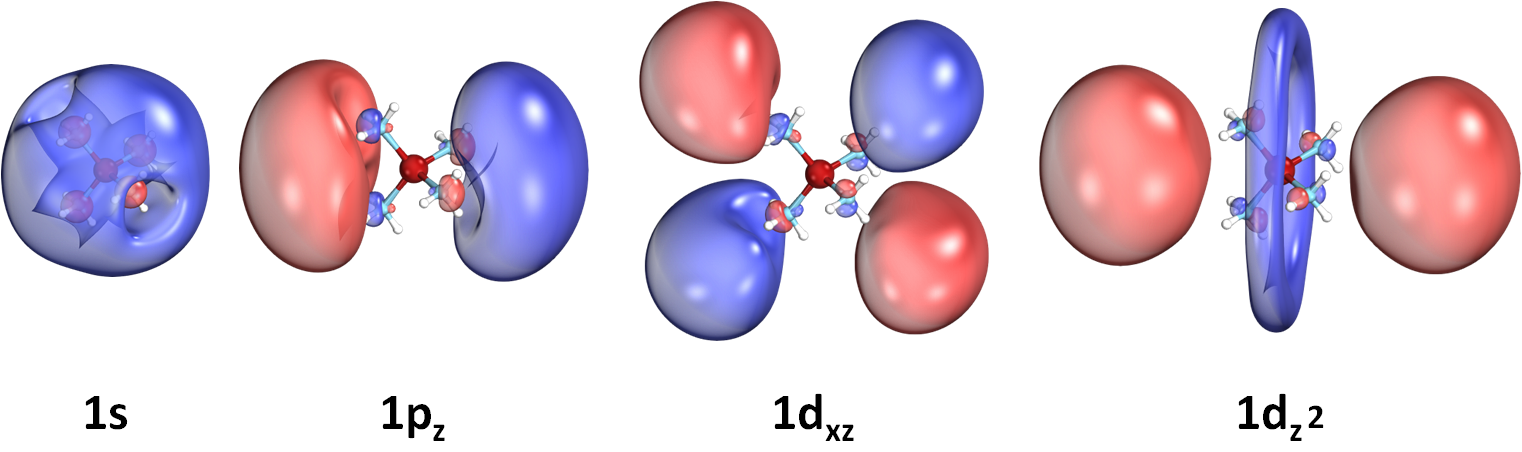
We have studied many more metals, such as Li, Na, Mg, Sc, V, and Y. All of them reveal unique features.
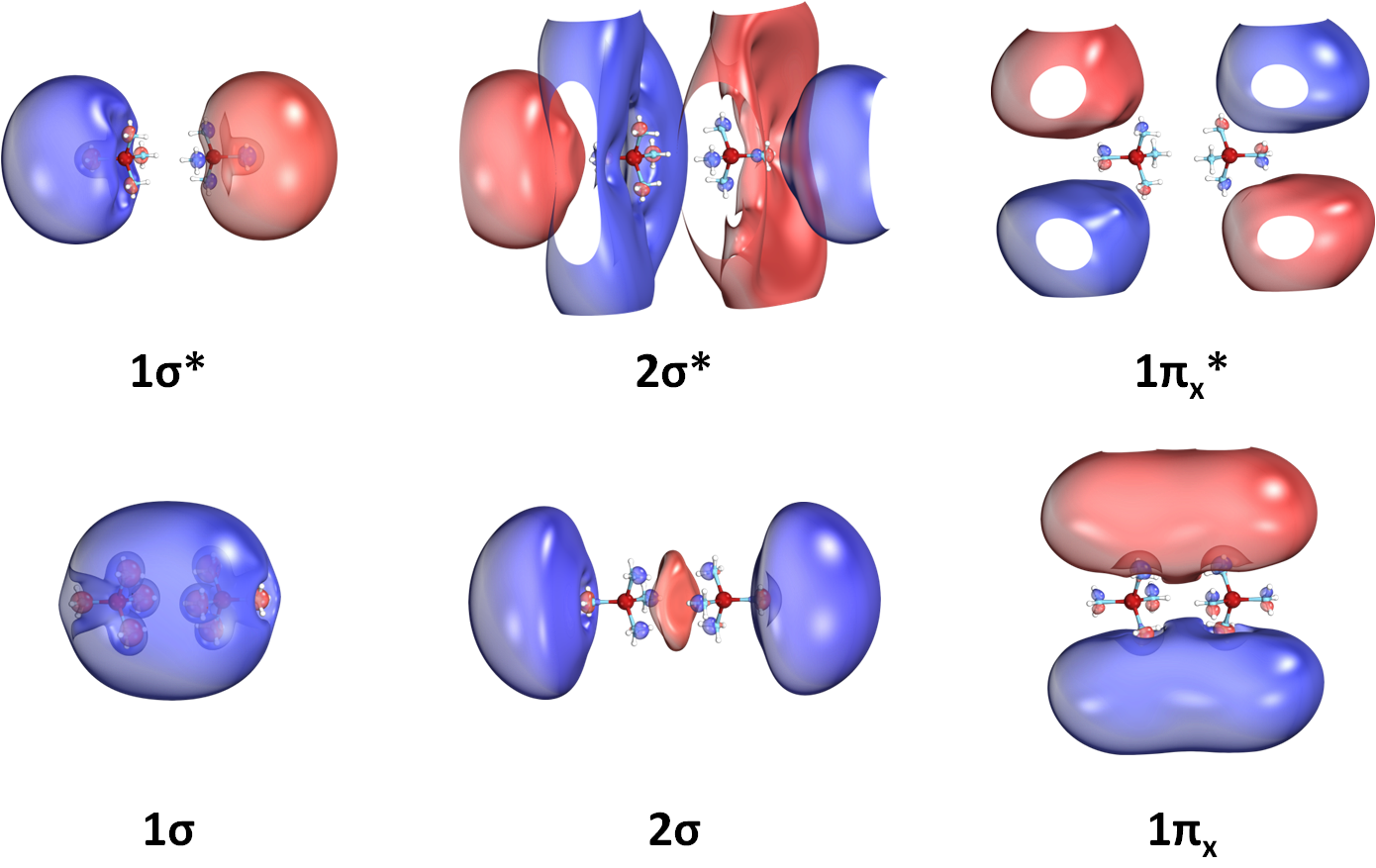
We have even seen that such molecular species can create dimers, exactly like two atoms make diatomic molecules.
The produced molecular orbitals have also character. See for example the orbitals of the Li(NH3)4 dimer:
SEP-polymers or even crystal structures of them are expected to form materials of unprecedented electronic and magnetic properties.
We propose novel materials that can be used for quantum computing and redox catalysis. We recently demonstrated that they can be used for CO2 capture and utilization!
Methane to methanol conversion and CO2 capture and utilization using metal anion complexes
Two very important processes is the selective conversion of methane to methanol and capture of carbon dioxide from the atmosphere.
The C-H bond of methane is one of the strongest C-H bonds and that of methanol is weaker and can be activated further.
How do we stop the reaction before methanol is overoxidized? Are there catalysts that can do this?
We have found that anionic species can actually do this. Currently, we are exploring different metals and ligands to optimize the efficiency of this conversion.
CO2 is an active green house gas and actually very harmful. We have found that metal anions can capture it and at the same time convert it to industirally valuable chemicals like lactones!
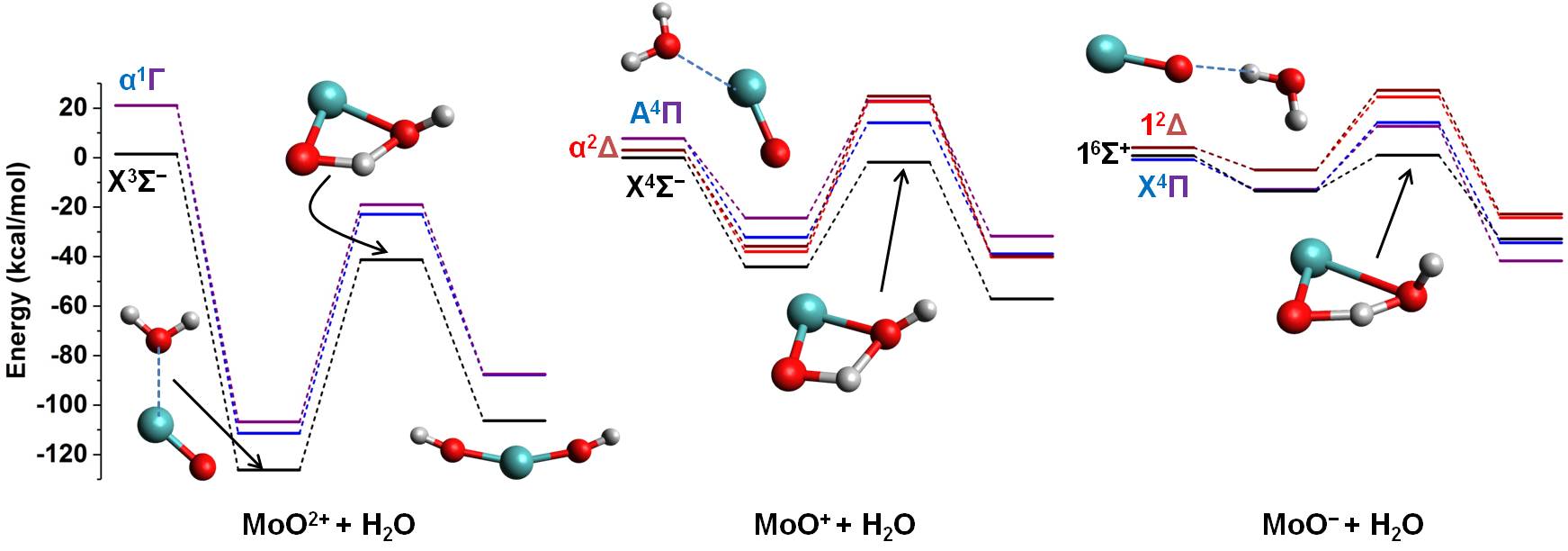
Water splitting
Molecular hydrogen is considered one of the future green fuels.
Its production from water is a promising approach with three main advantages:
1) water is abundant on earth
2) the process may be facilitated by solar light
3) the products, H2 and O2, can be recombined to produce energy.
Our goal is to use theoretical chemistry approaches to comprehend the fundamental principles guiding the chemistry
of the reaction 2H2O → 2H2 + O2 catalyzed by small transition metal compounds.
Specifically, what is the role of the metallic electronic structure? How the ligands of the metal affect the catalytic activity?
Which excited electronic states must be involved for the better exploitation of the soler light?
For example, we have found that water insertion in the first coordination sphere of the catalyst is more efficient
if the catalyst is negatively charged because it combines the low stability of the interacting complex of the reactants,
the small activation energy, and the legitimate stability of the products [DOI: 10.1039/C8CP01676C]: