IDEAL GAS LAWS
4E10.10 CHARLES'S LAW (T VS V)4E10.15 TEMPERATURE AND PRESSURE
4E10.20 BALLOONS IN LIQUID NITROGEN
4E20.20 BOYLE'S LAW (P VS V)
4E20.40 BALLOON EXPANSION IN VACUUM
|
4E10.10 Charles's Law (V ~ T) Temperature increase will result in volume increasing. Pressure remains constant at atmospheric plus pressure due to weight of plunger.
Setup Requirements: Some assembly required. . Open plastic clips. Push the plunger to the bottom. Attach metal cylinder to device. Put cylinder on hot plate.
Equations: PV = NkT
Safety Issues: Red hot hotplate. Keep plastic tube away from hot plate burner. |
|
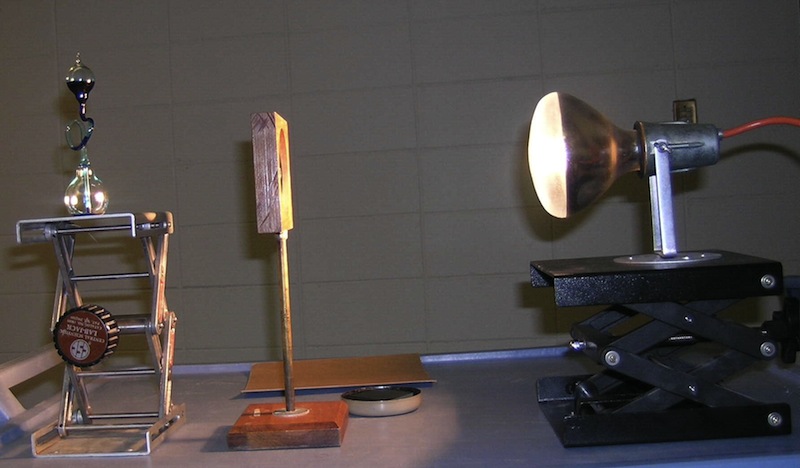
4E10.15 Temperature and Volume Focus bright light on bottom of device. The heat will cause the air volume to increase and force a colored liquid up a tube into top of device.
Setup Requirements: Ask ahead of time. Some assembly required.
Equations: PV = NkT
Safety Issues: hot light bulb, fragile glass tube |
|
4E10.20 Balloons in Liquid Nitrogen Put air filled balloon at top of larger container of LN. The volume will decrease as temperature decreases. May have to push it to get all the way in container. Remove and show volume increases while heating up.
Setup Requirements:Minimal. At least two working days notice or bring your own LN.
Equations: PV = NkT
Safety Issues: Liquid Nitrogen |
|
4E20.20 Boyle's Law (V and P) Demonstrate volume varies inversely with pressure. Add weights to top of device to increase pressure. The volume will decrease while temperature remains almost constant if done slowly. New device in picture on right will show temperature change on digital display if pressure increased rapidly. Setup Requirements: Some assembly required. Open plastic clips. Raise plunger to top. Close plastic clips. New version has screw device to lower plunger.
Equations: PV = NkT or nRT
Safety Issues: Dropped weights |
|
4E20.40 Balloon Expansion In Vacuum Blow up a balloon part way and put in a vacuum chamber. Attach vacuum pump and pump air out. As the pressure inside the chamber decreases the balloon will expand. This can also be done with marshmallows. Setup Requirements: Assembled as needed. Bring your own marshmallows. Equations: None Safety Issues: Imploding vacuum chamber. |