Roberts'
Lab Home Page
Sharon
R. Roberts
Associate Professor
Rm 127 Life Sciences Building
Department of Biological Sciences
Auburn University, AL 36849
Ph: (334)-844-1634, Fax: (334)-844-1645
e-mail: robersr@auburn.edu
Education:
|
B. A. |
Biology |
University of California, San
Diego |
|
M.P.H. |
Epidemiology |
University of California, Los
Angeles |
|
Ph.D. |
Microbiology |
University of California, Davis |
Research
Interests:
My research interests
are focused on host-parasite relationships and all of the research
projects in my laboratory address these interactions, although
in different systems and at different levels. Our research is
interdisciplinary and involves microbiology, molecular biology
and epidemiology.
Current projects in the
laboratory include:
1. Mycoplasmal conjunctivitis in the
house finch: Evolution of a new host-parasite relationship.
Mycoplasmal conjunctivitis is a new disease in the house finch. Since 1994, this infection has spread rapidly throughout the eastern range of the house finch causing a large reduction in this population. The disease was first observed in the Auburn area in 1995. In this collaboration with Dr. Geoff Hill, our two laboratories have the unique ability to assess a well-characterized wild population as well as to perform experimental 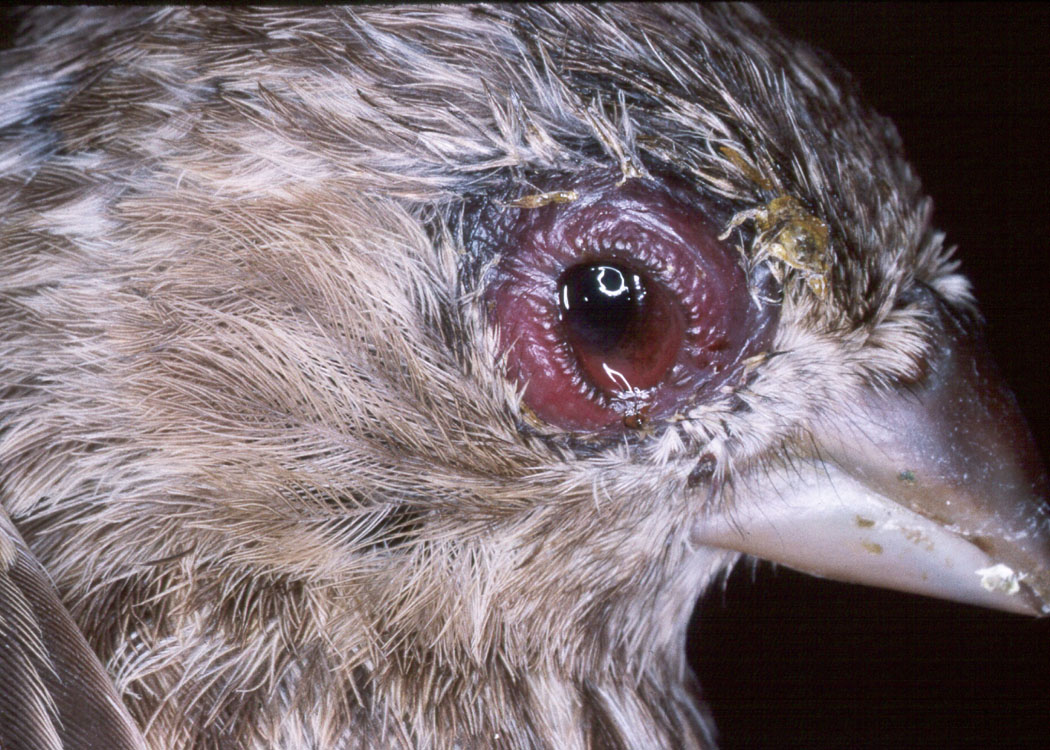 studies with captive birds providing the opportunity to analyze a rapidly evolving host-parasite relationship. We are assessing changes in virulence between early and late isolates of the pathogen as well as the susceptibility of naive house finch populations in the western United States to the infection. Through our collaboration with Dr. Scott Edwards at the University of Washington in Seattle we are characterizing the contribution of the host's genetic makeup to susceptibility to the mycoplasma.
studies with captive birds providing the opportunity to analyze a rapidly evolving host-parasite relationship. We are assessing changes in virulence between early and late isolates of the pathogen as well as the susceptibility of naive house finch populations in the western United States to the infection. Through our collaboration with Dr. Scott Edwards at the University of Washington in Seattle we are characterizing the contribution of the host's genetic makeup to susceptibility to the mycoplasma.
2. Attachment and entry of Respiratory Syncytial Virus
Very little is known about the interaction of RSV with the
host cell surface during virus attachment and entry. We are developing
two approaches to this problem.
a. We have developed a virus binding assay based on an ELISA and
are using this assay to characterize the initial interactions
between RSV and the cell surface. Most assays actually measure
binding indirectly by measuring infection. This assay provides
a direct measure of binding and will allow us to investigate the
role of the viral proteins in binding independent of their role
in the later steps of entry into the cell.
b. RSV is a difficult virus to work with in the laboratory because
of its unusual physical characteristics and these problems have
impeded studies on virus binding and entry. To facilitate the
study of the RSV envelope proteins in these processes, we are
using a virus-complementation assay utilizing a defective vesicular
stomatitis virus (VSV) that is unable to infect cells. VSV is
a much easier virus to study in the laboratory. This system will
allow us to test the contribution of the three RSV envelope proteins
to attachment and entry. This assay will then be used in subsequent
studies with altered forms of the RSV proteins to identify critical
regions involved in infection.
3. Mucosal immune response to Respiratory Syncytial Virus proteins
There is an increasing recognition of the importance of inducing
a local immune response to pathogens such as RSV which establish
infections on mucosal surfaces, however it has been very difficult
to design vaccines which induce a strong mucosal response and
this has hindered the development of an RSV vaccine. One alternative
is to combine immunologically important domains from viral proteins
with a mucosal adjuvant to enhance the local response.
We have constructed a number of genetic fusions that combine domains
of the RSV fusion protein and the mucosal adjuvant, the B subunit
of cholera toxin, and have expressed these recombinant proteins.
Currently, we are developing purification protocols for these
proteins. Subsequent studies will involve the characterization
of the immune response induced by this immunogen and its ability
to protect mice against challenge with wildtype RSV.
Recent Graduates:
Kaci Klenk M.S. Thesis project: "Development of a virus complementation system for the study of respiratory syncytial virus glycoprotein functions". Now working with the CDC on West Nile Virus in Colorado on a Public Health Laboratories Fellowship.
Shree Singh Ph.D. Dissertation project: "Development of a recombinant mucosal immunogen for respiratory syncytial virus". Faculty member at Alabama State University.
Current Students:
Kristy Farmer Ph.D. Dissertation project: "Suceptability of western house finches to mycoplasmal conjunctivitis".
Tim Oliver M.S. Thesis project: "Improved production of a recombinant mucosal immunogen for respiratory syncytial virus".
Publications:
Klenk, K. and Roberts, S. R. Use of a vesicular stomatis virus complementation system to analyze respiratory syncytial binding. Submitted. Virus Research.
Farmer, K. R., Hill, G. E., and Roberts, S. R. 2002. Susceptibility of a naive population of house finches to Mycoplasma gallisepticum. J. Wildlife Dis. 38:261-265.
Roberts, S. R., Nolan, P. M., Hill, G. E., 2001. Characterization of Mycoplasma gallisepticum infection in captive house finches (Carpodacus mexicanus) in 1998. Avian Dis. 45:70-75.
Roberts, S. R., Nolan, P. M.,
Lauerman, L. H., Li, L.-Q. and Hill, G. E. 2001. Characterization
of the mycoplasmal conjunctivitis epidemic in a southeastern house
finch (Carpodacus mexicanus) population. J. Wildlife Dis. 37:82-88.
Nolan, P. M., Duckworth, R. A., Hill, G. E. and Roberts, S. R.. In Press. Maintenance of a captive flock of house finches free of infection by Mycoplasma gallisepticum. Avian Dis. 44:948-952.
Piaskoski, T. O., Plumb, J.
A. and Roberts, S. R. 1999. Characterization of the largemouth
bass virus in cell culture. JAAH, 11:45-51.
Johnson, T. R., Johnson, J.
E., Roberts, S. R., Wertz, G. W., Parker, R. A., and Graham, B.
S. 1998. Priming with soluble glycoprotein G of respiratory syncytial
virus (RSV) augments interleukin-5 production and tissue eosinophilia
after RSV challenge. J. Virol., 72:2871-2880.
Furze, J., Roberts, S. R.,
Taylor, G. and Wertz, G. W. 1997. Antigenically distinct G glycoproteins
of BRSV strains share a high degree of genetic homogeneity. Virology,
231:48-58.
Current
Support:
NSF-"The Evolution
of a Parasite and its Recently Colonized Host", 2000-2004.
Co-P.I. with Dr. Geoff Hill (Auburn University) and Dr. Scott
Edwards (University of Washington, Seattle).
NIH-"Mucosal Immune Response to a RSV Protein Immunogen",
1999-2002.
Auburn University BioGrant-"Use of a Virus Complementation
System to Study Respiratory Syncytial Virus-Host Cell Interactions",
1999-2001
Teaching:
Immunology (BIOL 6500)
This course is offered in the Fall semester. BIOL 3000 and BIOL 3200 are prerequisites.
Techniques in Immunology
(BIOL 6501)
This is a hands-on
lab course which covers principles of immunology as well as a
number of widely used immunological methods and assays. BIOL 6500
is a prerequisite/corequisite for this course. It is offered in
the Fall semester.
Virology (BIOL 4230)
This course is offered
in the Spring semester. The prerequisites are BIOL 3000 and BIOL
3200.
Independent Research
Enthusiastic undergraduates interested in research opportunities
are most welcome in our laboratory. Independent research for undergraduates
provides experience with real science but it takes perserverance
and time. If you are interested, please make an appointment to
see me and we can discuss the current projects.
Back to the Department
of Biological Sciences Home Page
 studies with captive birds providing the opportunity to analyze a rapidly evolving host-parasite relationship. We are assessing changes in virulence between early and late isolates of the pathogen as well as the susceptibility of naive house finch populations in the western United States to the infection. Through our collaboration with Dr. Scott Edwards at the University of Washington in Seattle we are characterizing the contribution of the host's genetic makeup to susceptibility to the mycoplasma.
studies with captive birds providing the opportunity to analyze a rapidly evolving host-parasite relationship. We are assessing changes in virulence between early and late isolates of the pathogen as well as the susceptibility of naive house finch populations in the western United States to the infection. Through our collaboration with Dr. Scott Edwards at the University of Washington in Seattle we are characterizing the contribution of the host's genetic makeup to susceptibility to the mycoplasma.